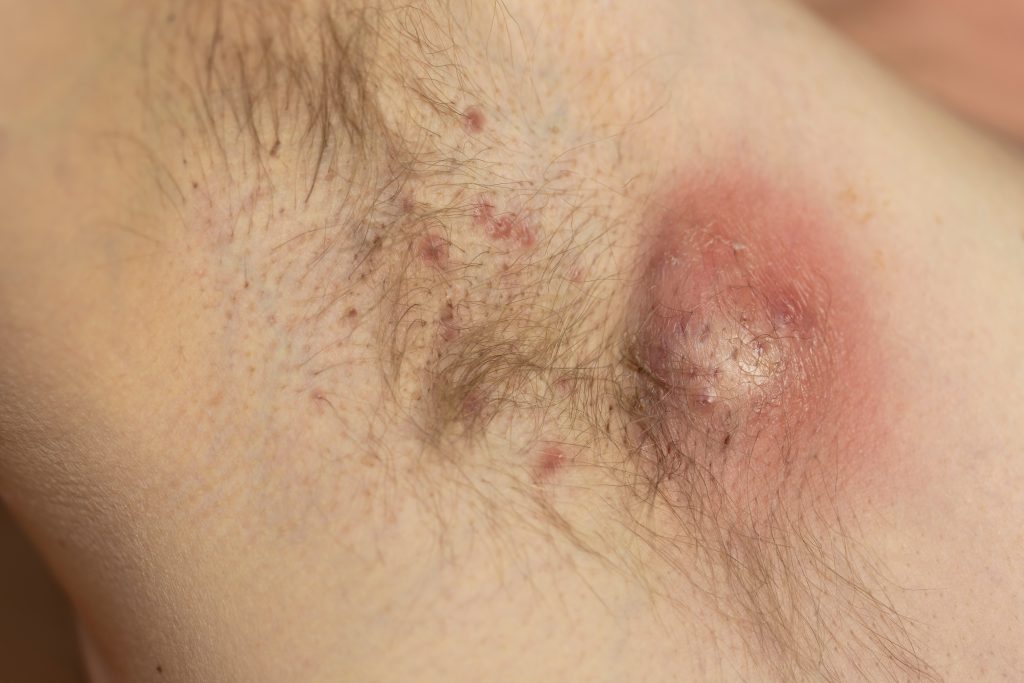
Remibrutinib, Novartis’ investigational oral Bruton’s tyrosine kinase (BTK) inhibitor, bested placebo in patients with moderate to severe hidradenitis suppurativa (HS), according to new data presented at the American Academy of Dermatology’s 2024 annual meeting in San Diego, CA.
B cells and plasma cells are involved in HS lesions, and BTK plays a role in the B-cell signaling path. Remibrutinib is also being studied in chronic spontaneous urticaria and multiple sclerosis. Only secukinumab (Cosentyx, Novartis) and adalimumab (Humira, AbbVie) have the U.S. Food and Drug Administration’s nod for HS, but the HS pipeline is bustling.
For the 16-week phase 2 trial study, researchers evaluated the safety and efficacy of remibrutinib in 77 adults with moderate to severe HS for at least 12 months in two or more anatomical areas with 15 or fewer tunnels beneath the skin.
Thirty-three patients were assigned to receive 100mg remibrutinib twice daily, 33 received a 25mg twice-daily dose, and 11 HS patients received placebo twice daily. The primary endpoint was the proportion of patients who achieved a simplified Hidradenitis Suppurativa Clinical Response (HiSCR) of at least a 50% reduction in total inflammatory abscess and nodule (AN) count with no increase in draining tunnels compared to baseline at week 16.
Fully 80.2% of patients completed the study. The main reason for discontinuing treatment was patient choice. Nearly 75% of patients in the remibrutinib 25mg twice-daily arm achieved the simplified HiSCR endpoint. By contrast, 48.5% of those in the remibrutinib 100 mg twice-daily arm, and 34.7% of those in the placebo arm hit this mark.
The researchers also reported that HiSCR, HiSCR 75, and HiSCR 90 rates were higher at week 16 among patients in both remibrutinib treatment arms compared with placebo. Moreover, remibrutinib was associated with a greater reduction of the AN count and draining tunnels than placebo. The estimated mean percentage reduction in AN count was 68% in the 25 mg twice-daily arm, 57% in the 100 mg twice-daily arm, and 49.7% in the placebo arm. Meanwhile, the estimated mean reductions in draining tunnels were 55.6%, 43.6%, and 10.2%, respectively, across the three arms, the study showed.
Patients treated with remibrutinib also showed a greater response on the Patient’s Global Assessment of Skin Pain Numeric Rating Scale 30 (NRS30) compared with those on placebo at week 16.
Adverse events (AEs) were mainly mild or moderate in severity. Infections were the most common AEs across all treatment arms.
The new study was presented by Alexa B. Kimball, MD, MPH, President and Chief Executive Officer of Harvard Medical Faculty Physicians at BIDMC, Inc in Boston and a Professor of Dermatology at Harvard Medical School.