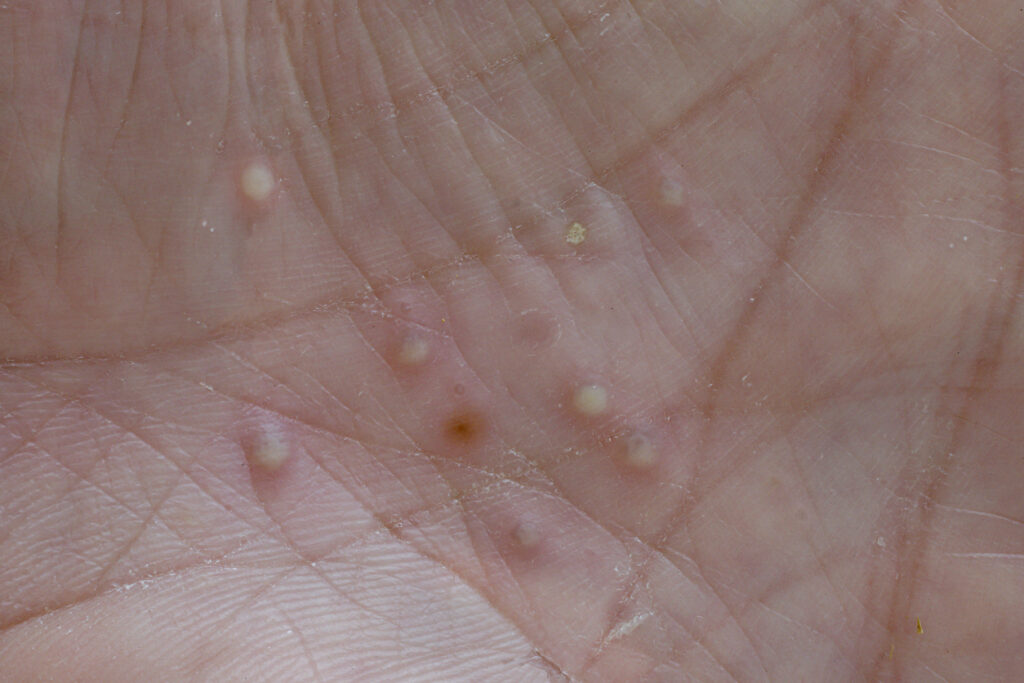
Apremilast (Otzela, Amgen) can improve symptoms in patients with moderate to severe palmoplantar pustulosis who had an inadequate response to topical therapy, according to a late-breaking Phase 3 study presented at the annual meeting of the American Academy of Dermatology in San Diego, CA.
The randomized, placebo-controlled, double-blind study included 176 patients who received apremilast (n=88) or placebo (n=88) for 16 weeks. Fully 67.8% of patients who received apremilast achieved the primary endpoint of Palmoplantar Pustulosis Area and Severity Index (PPPASI) 50 (>50% improvement in PPPASI); this was a significantly greater response as compared to placebo.
There were statistically significant improvements in all secondary endpoints observed with apremilast relative to placebo, including changes from baseline to week 16 in PPPASI, Palmoplantar Pustulosis Severity Index (PPSI), Patient Visual Analogue Scale of palmoplantar pruritus and pain/discomfort and Dermatology Life Quality Index(DLQI). Treatment-emergent AEs were consistent with the known safety profile of Otezla. The most common AEs throughout the study were diarrhea, soft feces, headache and nausea.
“Palmoplantar pustulosis can severely impact daily functioning due to its painful effects on the hands and feet, necessitating new therapeutic options for this impactful inflammatory skin condition,” says Melinda Gooderham, M.D., FRCPC, dermatologist and clinical investigator at SKiN Centre for Dermatology and assistant professor, Queen’s University, Ontario, Canada, in a news release. “The study presented at AAD highlighted significant improvement in disease severity, symptoms such as itch, pain, discomfort, and patient-reported quality of life among those treated with Otezla compared to placebo.”