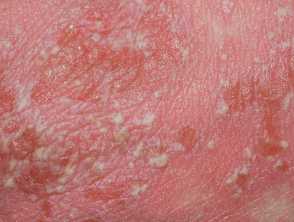
The U.S. Food and Drug Administration (FDA) has approved spesolimab-sbzo (Spevigo, Boehringer Ingelheim) injection for the treatment of generalized pustular psoriasis (GPP) in adults and pediatric patients aged 12 and above weighing ≥40 kg.
This approval follows the Chinese National Medical Products Administration’s (NMPA) recent approval of spesolimab-sbzo for the reduction of occurrence of GPP in adolescents starting at 12 with a body weight ≥40 kg and adults.
Spesolimab-sbzo is a novel, humanized selective IgG1 antibody that binds to interleukin-36 receptor (IL-36R). It was previously approved for adults with GPP.
The regulatory authorities’ decisions are based on the positive results of the Effisayil 2 clinical trial, a 48-week clinical trial that showed that spesolimab-sbzo significantly reduced the risk of GPP flares by 84%, compared with placebo. In the trial with 123 patients, no flares were observed after week 4 of spesolimab-sbzo subcutaneous treatment in the high-dose group (n=30). In the Effisayil 2 trial, spesolimab-sbzo was associated with an increased incidence (≥9 cases per 100 patient-years) of injection site reaction, urinary tract infection, arthralgia, and pruritus compared to placebo.
“Until now, people living with GPP have not had any approved options to treat their disease,” says Dermatology Digest Editorial Advisory Board Member Bruce Strober, MD, PhD, Clinical Professor, Dermatology, Yale University and Central Connecticut Dermatology, in Cromwell, CT, in a news release. “Spesolimab-sbzo has the potential to redefine the treatment options for the patients we serve.”
Carinne Brouillon, Member of the Board of Managing Directors and Head of Human Pharma at Boehringer Ingelheim, adds, “Experiencing GPP can be mentally and physically devastating, leaving those affected with uncertainty and fear of the next episode. Therefore, expanding the treatment of GPP is a critical step towards addressing patients’ needs.”
PHOTO CREDIT: DermNet NZ