EMA Oks Soligenix’s Second Confirmatory Phase 3 Trial for HyBryte in CTCL
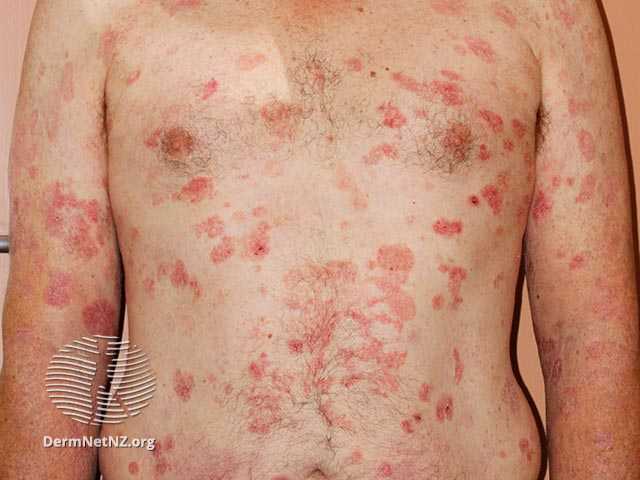
Soligenix, Inc. has gotten a green light from the European Medicines Agency (EMA) on the key design components of a confirmatory Phase 3 placebo-controlled study evaluating the safety and efficacy of synthetic hypericin (HyBryte) for the treatment of cutaneous T-cell lymphoma (CTCL) patients with early-stage disease. HyBryte (SGX301) is a novel, first-in-class, photodynamic therapy utilizing […]
