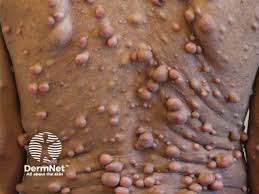
The European Commission (EC) granted conditional marketing authorization for mirdametinib (Ezmekly, SpringWorks Therapeutics, Inc.) for the treatment of symptomatic, inoperable plexiform neurofibromas (PN) in pediatric and adult patients with neurofibromatosis type 1 (NF1) aged two and older.
Mirdametinib is the first and only therapy approved in the European Union (EU) for both adults and children with NF1-PN.
“Patients with NF1-PN often face physical and mental health challenges and impaired quality of life given the limited treatment options available for this lifelong and debilitating disease,” says Ignacio Blanco, MD, PhD, Chairman of the National Reference Center for Adult Patients with Neurofibromatosis at Hospital Universitari Germans Trias I Pujol in Barcelona, Spain, in a news release. “This approval represents an important advance, especially for adults who previously did not have an approved treatment. In clinical trials, EZMEKLY demonstrated an encouraging efficacy and safety profile in both adults and children, and importantly, is available in a tablet that dissolves easily in water for people who are unable to swallow a pill and could therefore not previously receive therapy.”
An Important Milestone
“This European Commission approval is an important milestone for NF patients and caregivers, as it means more treatment options for patients with plexiform neurofibromas, including adults,” adds Annette Bakker, PhD, Chief Executive Officer of the Children’s Tumor Foundation (CTF), and Dariusz Adamczewski, MD, Director CTF Europe. “This is the kind of progress that happens when researchers, industry, and organizations like ours work together with a shared focus on delivering new treatments for patients.”
The EC approval of mirdametinib is based on results from the ongoing, multi-center, open-label, single-arm Phase 2b ReNeu trial, which enrolled 114 patients with NF1-PN age 2 years or older (58 adults and 56 pediatric patients). The study met the primary endpoint of confirmed objective response rate (ORR), as assessed by blinded independent central review, demonstrating an ORR of 41% (N = 24/58) in adults and 52% in children (N = 29/56). The median best percentage change in target PN volume was -41% (range: -90 to 13%) in adults and -42% (range: -91 to 48%) in children.
Among those with a confirmed response, 88% percent of adults and 90% of children had a response of at least 12 months’ duration, and 50% and 48%, respectively, had a response of at least 24 months’ duration. Both adults and children also experienced early and sustained significant improvements from baseline in pain and quality of life as assessed across multiple patient-reported outcome tools.
Mirdametinib demonstrated a manageable safety and tolerability profile. The most common adverse reactions reported in adults receiving EZMEKLY were dermatitis acneiform (83%), diarrhea (55%), nausea (55%), increased blood creatine phosphokinase (47%), musculoskeletal pain (41%), vomiting (37%), and fatigue (36%). The most common adverse reactions occurring in children were increased blood creatine phosphokinase (59%), diarrhea (53%), dermatitis acneiform (43%), musculoskeletal pain (41%), abdominal pain (40%), vomiting (40%), and headache (36%).
PHOTO CREDIT: DermNet