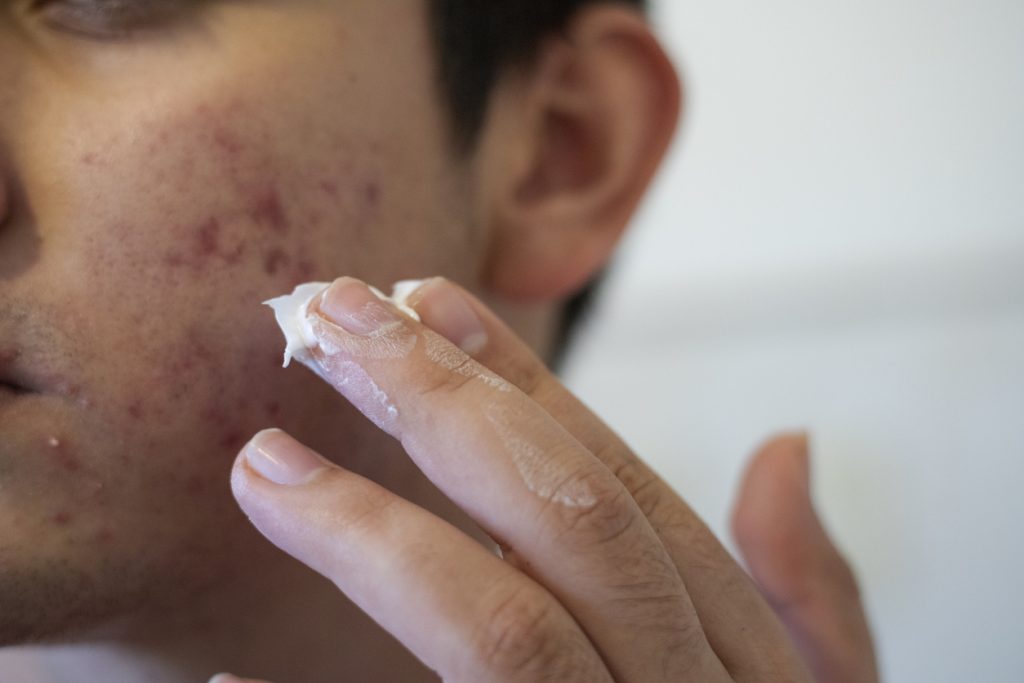
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved clascoterone cream 1% (Winlevi, Cosmo and Glenmark) for acne vulgaris in patients aged 12 years and older in the UK.
Clascoterone cream 1% is the first commercially available topical androgen receptor inhibitor. This approach is believed to help reduce sebum production and intervene early in acne pathogenesis. Two identical phase III studies demonstrated that clascoterone cream 1%, applied topically twice daily for 12 weeks, was more effective than the application of vehicle cream in achieving the Investigator’s Global Assessment (IGA) of success, reducing non-inflammatory lesion count (NILC) and inflammatory lesion count (ILC) in patients with facial acne vulgaris. clascoterone cream 1%, was generally well tolerated.
As part of the agreement signed in September 2023, Glenmark has in-licensed the product from Cosmo Pharmaceuticals for distribution in Europe and South Africa. Clascoterone cream 1% has been approved by the United States Food & Drug Administration (U.S. FDA) for the topical treatment of acne in patients aged 12 years and older. Sun Pharma launched Clascoterone cream 1%in the US market in November 2021.