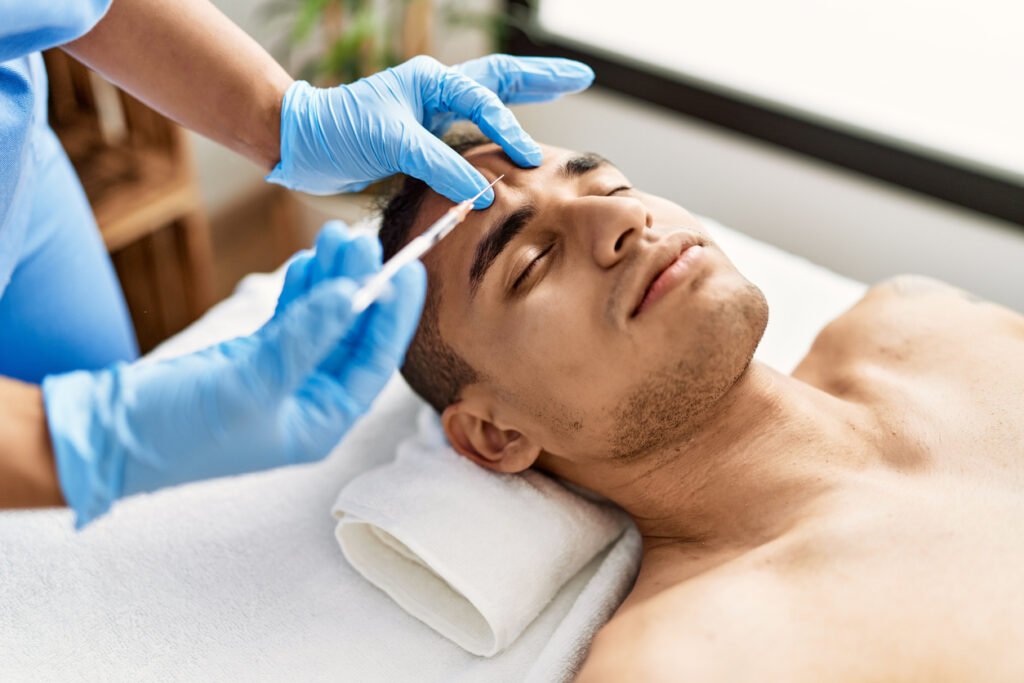
The U.S. Food and Drug Administration (FDA) sent warning letters to 18 websites that illegally market unapproved and misbranded botulinum toxin products.
The FDA is aware of adverse events associated with these botulinum toxin products, including botulism symptoms.
“Unapproved and misbranded Botox products carry serious health risks. Today, we’re taking action to protect American consumers and prevent online entities from selling these dangerous products,” says FDA Commissioner Marty Makary, MD, MPH, in a news release.
The letters were addressed to:
- acecosm.com
- aesthetic-essentials.com
- celestapro.com
- cosmenic.net
- cosmo-korea.com
- derma-solution.com
- dermaxshop.com
- ellepharm.com
- estaderma.com
- filleroutlet.com
- glamderma.com
- glowface.store
- glownestbeauty.com
- koreafillerexperts.com
- koreanfillers.com
- maypharm.net
- meamoshop.com
- mjsmedicals.com
The sites are based in South Korea, China, Panama, and the US.
Healthcare professionals and consumers should report any adverse events with these products to MedWatch.