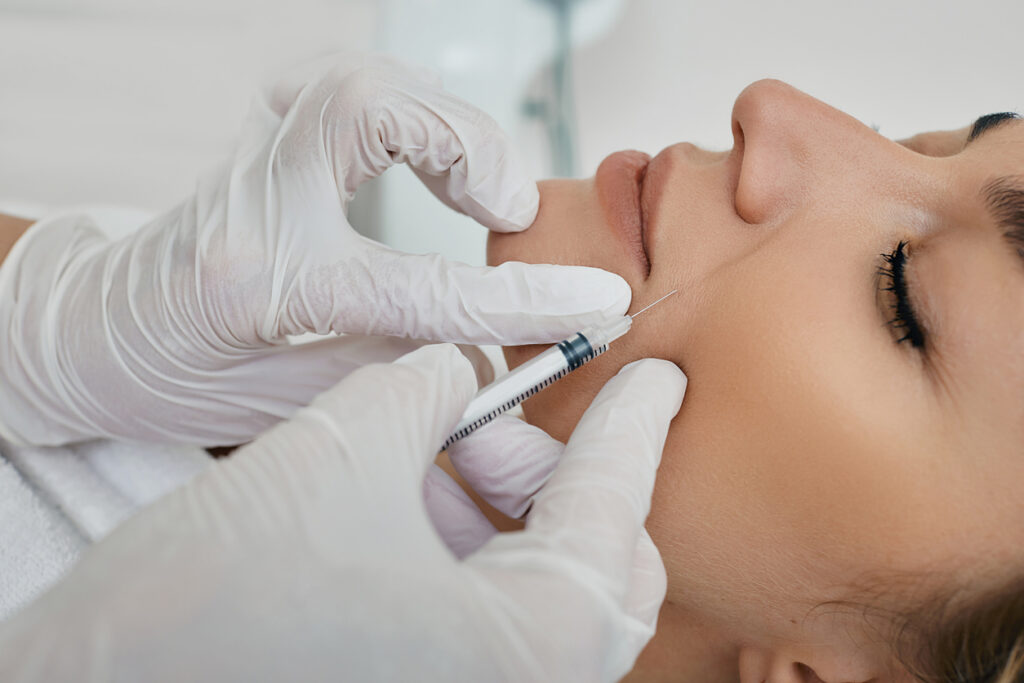
The U.S. Food and Drug Administration (FDA) has approved Obagi saypha MagIQ injectable hyaluronic acid (HA) gel, the first intradermal filler product in the Obagi saypha collection for commercial use in the U.S. market.
The filler was developed by Croma–Pharma and brought to the U.S. through its strategic partner Waldencast plc under the Obagi Medical brand. The commercial launch is planned for 2026 and represents the beginning of a multi-product U.S. rollout that will also include saypha ChIQ and additional products. The new filler utilizes proprietary MACRO Core Technology that creates a stable 3D HA matrix for natural-looking results and boasts consistent gel distribution and a predictable injection force and swelling profile.
Approval for Obagi saypha MagIQ is based on a pivotal study for nasolabial fold (NLF) correction including 270 patients and confirming non-inferiority versus the FDA approved control product, meeting the primary performance endpoint with key secondary endpoints further. The trial included all Fitzpatrick Skin Types demonstrating the product’s effectiveness and safety across a representative population.
“The FDA approval of Obagi saypha MagIQ reflects our commitment to bringing high-quality, science-backed aesthetic products to market,” says Suzan Obagi, MD, Chief Medical Director of Obagi Medical, in a news release/ This addition to our portfolio reinforces Obagi Medical’s dedication to the highest standards of patient safety and efficacy. With the introduction of its MACRO Core Technology, Obagi saypha MagIQ offers a differentiated approach to the dermal filler category, providing healthcare practitioners with new options to address the unique needs of their patients with precision and confidence across all skin types and tones.”
Jeremy Green, MD, Investigator for the U.S. NLF pivotal study, adds, “Obagi saypha MagIQ demonstrated impressive safety, efficacy, and versatility in the trial, with smooth and consistent injection properties. This product allows practitioners to achieve precise, natural-looking results while delivering high patient satisfaction with its effectiveness and longevity. I look forward to utilizing it in my practice in the near future.”