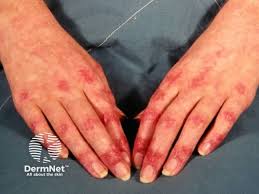
The U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for Restem’s umbilical cord outer lining stem cells (ULSCs) program for the treatment of dermatomyositis (DM) and polymyositis (PM).
Restem’s ULSC therapy has shown promising results in early clinical trials, demonstrating safety, tolerability and initial clinically significant improvements, as well as the potential to significantly reduce the need for steroid use in patients. The company is currently preparing for Phase 2/3 trials with anticipated initiation in 1Q 2025.
Both disorders can significantly compromise quality of life and are very challenging to treat. The estimated incidence of PM/DM is approximately 5 to 22 cases per 100,000 individuals. Current treatments include long-term use of corticosteroids and immunosuppressive therapies, which can be associated with significant toxicity and other side effects.
The FDA’s Office of Orphan Products Development grants ODD status to drugs and biologics intended for the safe and effective treatment, diagnosis, or prevention of rare diseases or conditions affecting fewer than 200,000 people in the United States. ODD provides benefits to drug developers designed to support the development of drugs and biologics for small patient populations with unmet medical needs. These benefits include assistance in the drug development process, tax credits for qualified clinical costs, exemptions from certain FDA fees, and seven years of marketing exclusivity.
“Receiving the Orphan Drug Designation (ODD) is a significant milestone in our mission to provide effective and safer treatment options for patients suffering from PM/DM,” says Keith March, MD, PhD, Chief Medical Officer of Restem, in a news release. “We believe that our novel ULSC therapy has the potential to offer meaningful benefits for patients by modulating the immune system, which may reduce the reliance on steroids. We look forward to the continued development of the ULSC platform and working closely with regulators to bring its potential to patients in need.”
PHOTO CREDIT: DermNet