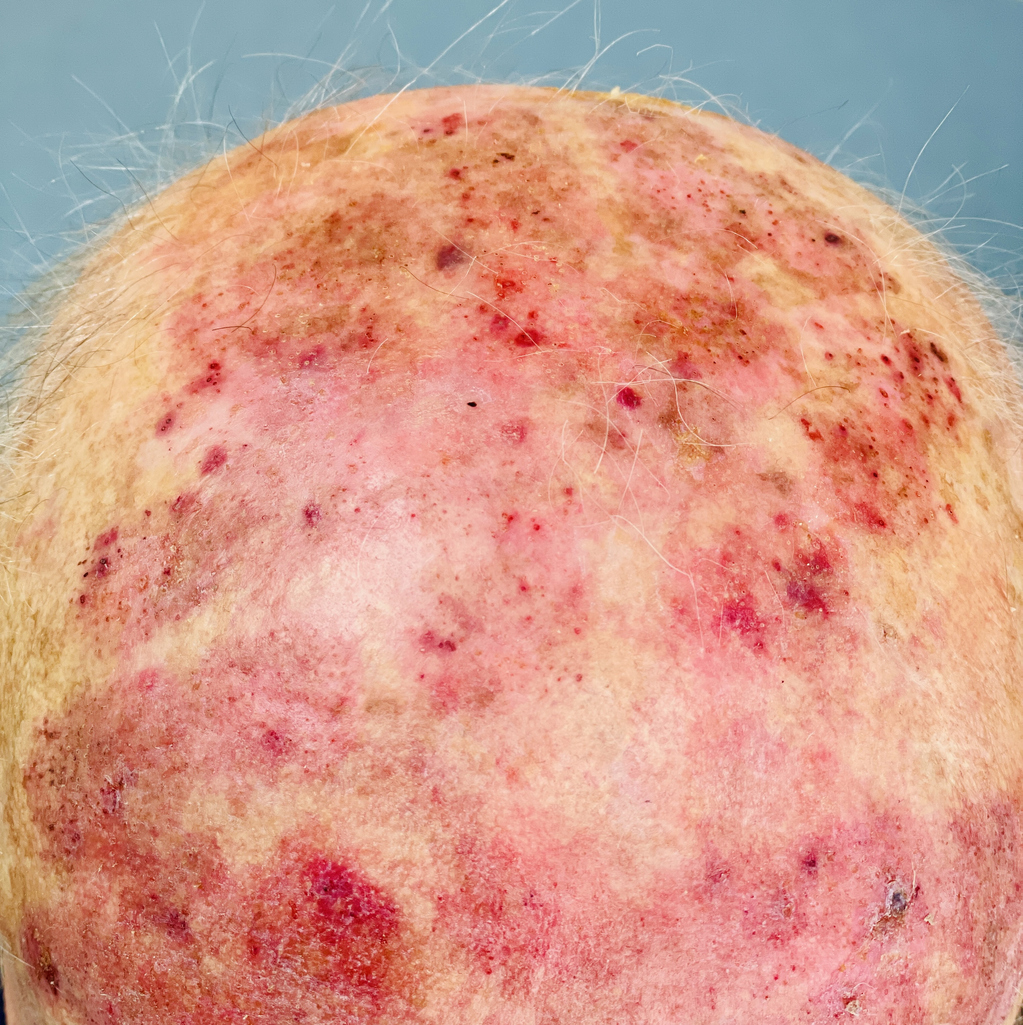
The U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for a Phase 1b/2a study of Rubedo Life Sciences’ RLS-1496 in patients with actinic keratosis (AK).
RLS-1496 is a first-in-class disease-modifying selective glutathione peroxidase 4 (GPX4) modulator targeting pathological senescent cells that drive inflammaging and chronic degenerative diseases and conditions associated with the biological aging process.
The study is expected to begin in Q4 2025. The first European Medicines Agency-cleared RLS-1496 clinical trial began in May 2025 for plaque psoriasis, atopic dermatitis, and skin aging; study results are expected in Q4 2025.
Rubedo’s proprietary, artificial intelligence (AI)-driven drug-discovery platform Alembic identifies cellular and molecular targets for pathologic senescent cells and develops selective medicines that restore tissue homeostasis for these targets.
“This second IND clearance supports our focus on the potential of RLS-1496 as the first-ever GPX4 modulator to treat an array of age-related diseases and conditions, which will allow us to have the greatest impact for patients,” says Rubedo CEO Frederick Beddingfield, III, MD, PhD, FAAD, FACMS, in a news release.
Rubedo Chief Scientific Officer Marco Quarta, PhD, adds, “This IND clearance comes just four months after the initiation of a Phase 1 study with RLS-1496 in patients with psoriasis in Europe. We are excited to begin this new trial and showcase the breadth of therapeutic impact that can be made by targeting inflammatory and aging mechanisms like GPX4.”
Emma Guttman-Yassky, MD, PhD, Joins CAB
In related news, Rubedo has also welcomed Emma Guttman-Yassky, MD, PhD, Waldman Professor and Chair of the Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, NY, to its Clinical Advisory Board (CAB). She is also the Director of the Occupational Dermatitis Clinic and Director of the Laboratory for Inflammatory Skin Diseases.
Dr. Guttman says, “Millions of people are affected by dermatologic inflammatory skin conditions, including psoriasis and actinic keratosis. As a researcher and clinician, I am inspired by cutting-edge longevity science and recognize the need for new innovations that can improve patient health. This IND clearance is an important step forward in making these types of innovations a reality, and I am delighted to be working with the Rubedo team at this exciting time.”