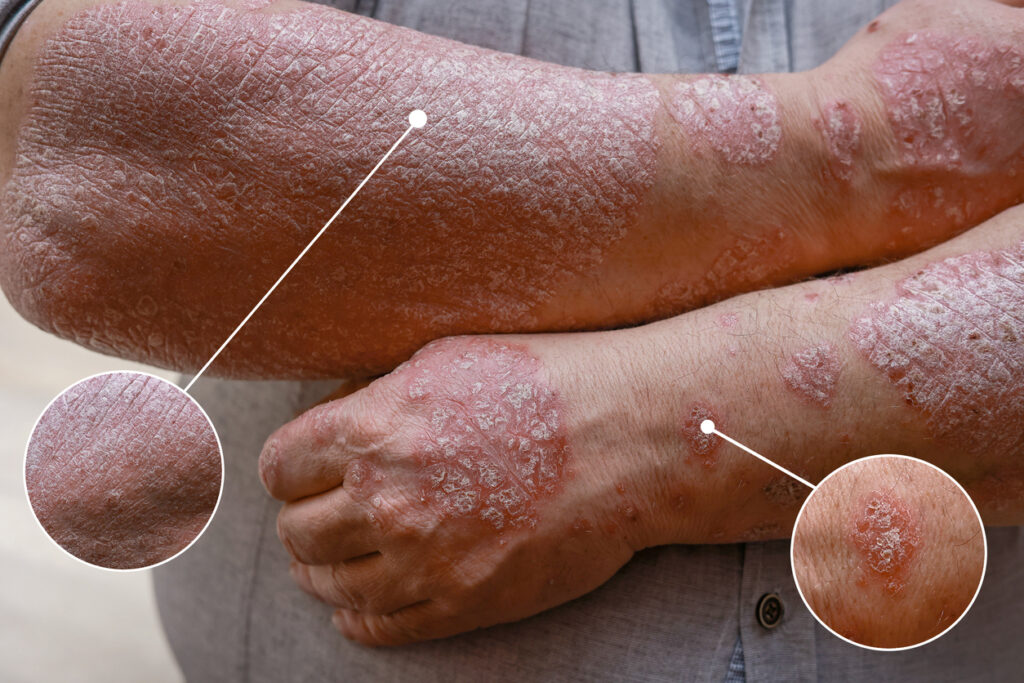
The U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application for a Phase I trial for Ascletis Pharma Inc.’s ASC50 for the treatment of mild-to-moderate plaque psoriasis.
ASC50 is an oral small molecule inhibitor targeting interleukin-17 (IL-17).
Following oral dosing in non-human primates, ASC50 demonstrated higher drug exposure, longer half-life, and lower clearance than an oral small molecule IL-17 inhibitor comparator, which is currently in the clinical development. Furthermore, ASC50 demonstrated strong efficacy in a psoriasis animal model. These preclinical data support ASC50 as a potential best-in-class once-daily oral drug candidate for the treatment of psoriasis.
The Phase I clinical trial of ASC50 is a randomized, double-blind, placebo-controlled study and will be conducted at multiple sites in the U.S. Dosing of patients with mild-to-moderate plaque psoriasis is expected to start in the third quarter of 2025.
“We are excited and encouraged by the preclinical data of ASC50 as it is the first oral small molecule drug candidate in immunology arisen from our Artificial Intelligence-assisted Structure-Based Drug Discovery (AISBDD) Platform,” says Jinzi Jason Wu, PhD, Founder, Chairman and CEO of Ascletis, in a news release. “The IND clearance of ASC50 marks a new milestone for Ascletis in autoimmune and inflammatory diseases. We are continuing to work on differentiated agents including oral drugs and once monthly or less frequent subcutaneously injectables to address unmet medical needs in multiple key therapeutics areas.”