Add the treatment of adults with bullous pemphigoid (BP) to the long list of U.S. Food and Drug Administration (FDA) approvals for dupilumab (Dupixent, Sanofi & Regeneron).
Dupilumab is the first and only targeted medicine to treat BP in the U.S. It is already approved for certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, prurigo nodularis, chronic spontaneous urticaria, and chronic obstructive pulmonary disease in different age populations.
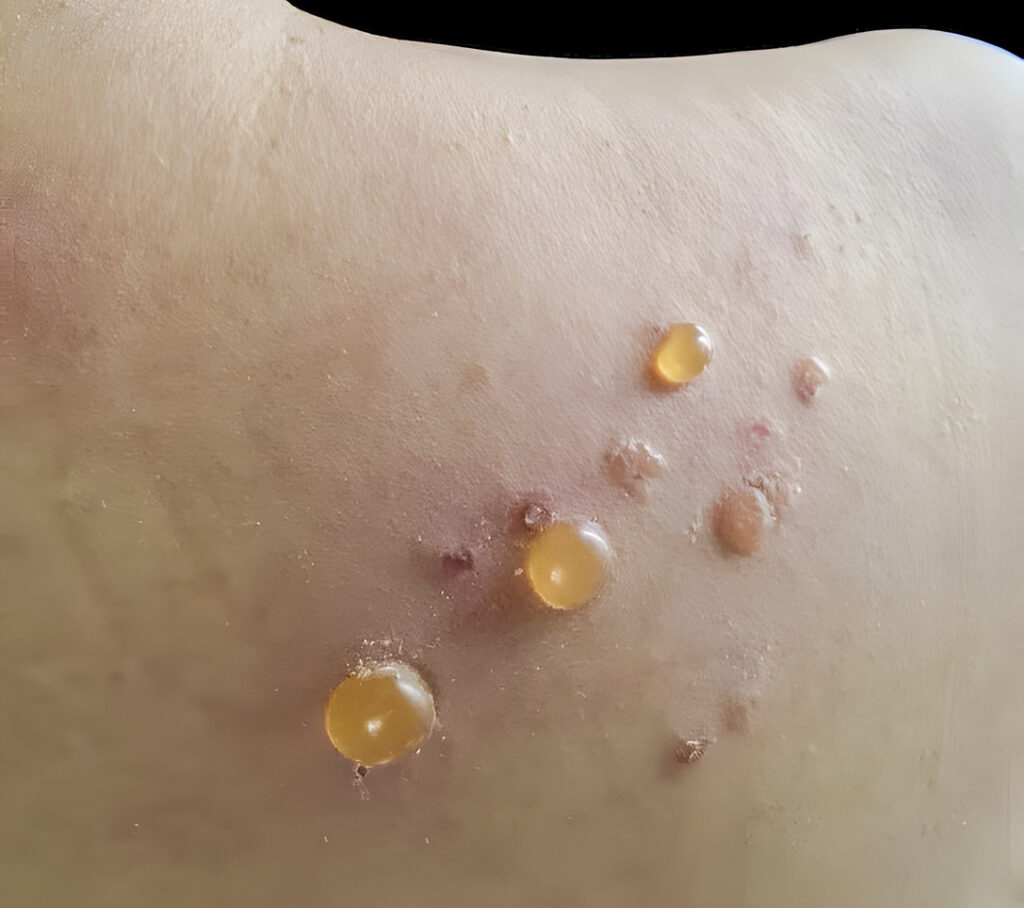
“The medications that we use to treat #bullouspemphigoid can be as bad as the disease itself,” says Eingun James Song, MD, FAAD, Co-Chief Medical Officer and Director of Clinical Research at Frontier Dermatology in Seattle, WA. “For a disease where patients are older, sicker, and often on dozens of other medications, safety is paramount. Like many of you, I’ve been using #dupixent for years off-label to treat this condition with great success. Now it’s officially on-label.”
Adam Friedman, MD, Professor and Chair of Dermatology at the George Washington University School of Medicine & Health Sciences in Washington, D.C., is also excited about the FDA nod.
No Longer an Orphan
“Even though BP is still an orphan disease, it is an orphan no longer with respect to having the first FDA-approved treatment for this uniquely disabling condition that has its sights on a fragile and susceptible population: the elderly,” says Dr. Friedman. “Even thinking about the off-label use of medications like systemic steroids and traditional immunosuppressants comes with a lot of baggage, especially for a patient population that has multiple comorbidities and polypharmacy, which makes managing this itchy, destructive condition quite challenging.”
Dr. Friedman continues, “Having an option like dupilumab not only provides a safe and effective option as proven through an FDA trial pipeline but also provides us with unique insight on how a medication like this behaves in an older population that is not typically included in clinical trials.”
ADEPT Trial Details
The approval is supported by data from the pivotal ADEPT Phase 2/3 study that evaluated the efficacy and safety of dupilumab compared to placebo in adults with moderate-to-severe BP. Patients were randomized to receive dupilumab 300mg (n=53) or placebo (n=53) added to standard-of-care oral corticosteroids (OCS). During treatment, all patients underwent a protocol-defined OCS tapering regimen if control of disease activity was maintained. During the FDA review, the analyses were updated; the FDA-approved results at 36 weeks in the label for dupilumab compared to placebo are:
- 18.3% of patients experienced sustained disease remission compared to 6.1% (12.2% difference; 95% confidence interval: -0.8% to 26.1%), the primary endpoint
- 38.3% of patients achieved clinically meaningful itch reduction compared to 10.5%
- Median cumulative OCS dose was 2.8 grams compared to 4.1 grams
In this elderly population, the most common adverse events (≥2%) more frequently observed in patients on Dupixent compared to placebo were arthralgia, conjunctivitis, blurred vision, herpes viral infections, and keratitis. Additionally, one case of acute generalized exanthematous pustulosis was reported in one patient treated with dupilumab and zero patients treated with placebo.
Dupilumab was granted Priority review from the FDA due to its potential to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. Dupilumab was previously granted orphan drug designation by the FDA for BP, which applies to investigational medicines intended for the treatment of rare diseases that affect fewer than 200,000 people in the U.S.