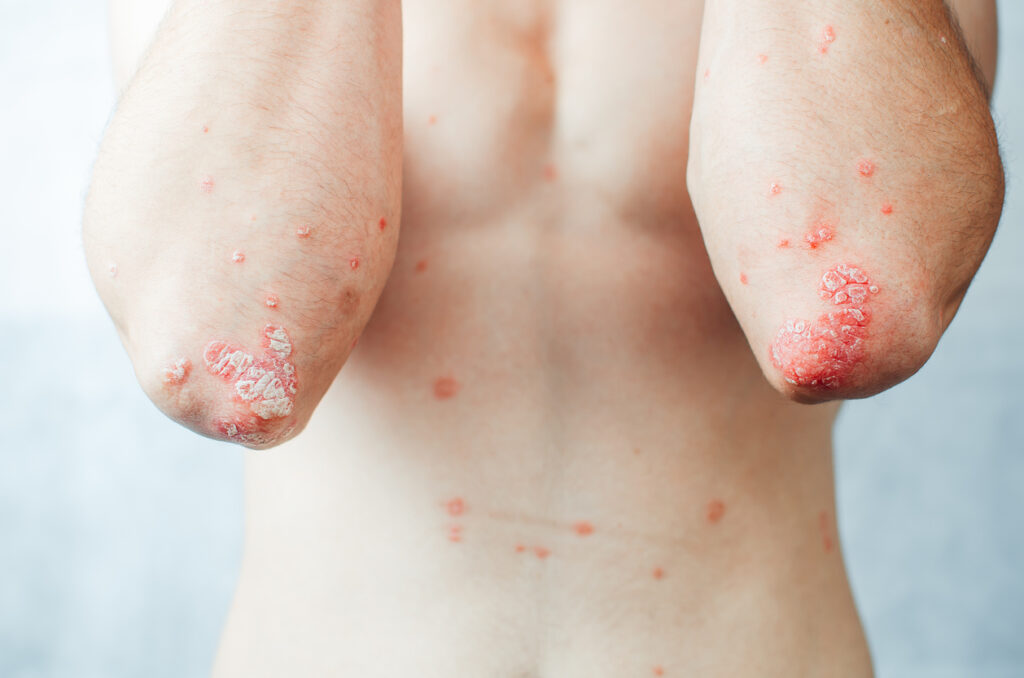
The U.S. Food and Drug Administration (FDA) has approved a new presentation of Celltrion, Inc’s Stelara (ustekinumab) biosimilar.
Steqeyma is now available in a 45mg/0.5mL solution in a single-dose vial for subcutaneous injection. The additional presentation is approved for the treatment of pediatric patients aged 6 to 17 years, weighing less than 60kg, with plaque psoriasis or psoriatic arthritis.
In December 2024, the FDA approved Steqeyma in 45mg/0.5mL and 90mg/mL solutions in a single-dose prefilled syringe for subcutaneous injection, and 130mg/26mL in a single-dose vial for intravenous infusion in adult and pediatric patients 6 years and older with plaque psoriasis and psoriatic arthritis, as well as adult patients with Crohn’s disease and ulcerative colitis.
“Managing inflammatory diseases in pediatric patients can be particularly complex,” says Hetal Patel, PharmD MBA, Vice President of Medical Affairs at Celltrion USA, in a news release. “The new dosage form and strength of STEQEYMA allow us to better meet the specific needs of young patients, giving physicians a valuable treatment option with flexibility, supported by a well-established safety and efficacy profile.”
The FDA approval of Steqeyma was based on the totality of evidence, including the results from a Phase 3 study in adults with moderate to severe plaque psoriasis, in which the primary endpoint was the rate of change in the Psoriasis Area and Severity Index (PASI) for skin symptoms. The clinical results demonstrated that Steqeyma and its reference product, ustekinumab, are highly similar, and have no clinically meaningful differences in terms of safety and efficacy.
The FDA has granted Steqeyma full interchangeability with Stelara across all indications of Stelara, following the expiration of exclusivity for the first interchangeable biosimilar on April 30, 2025.