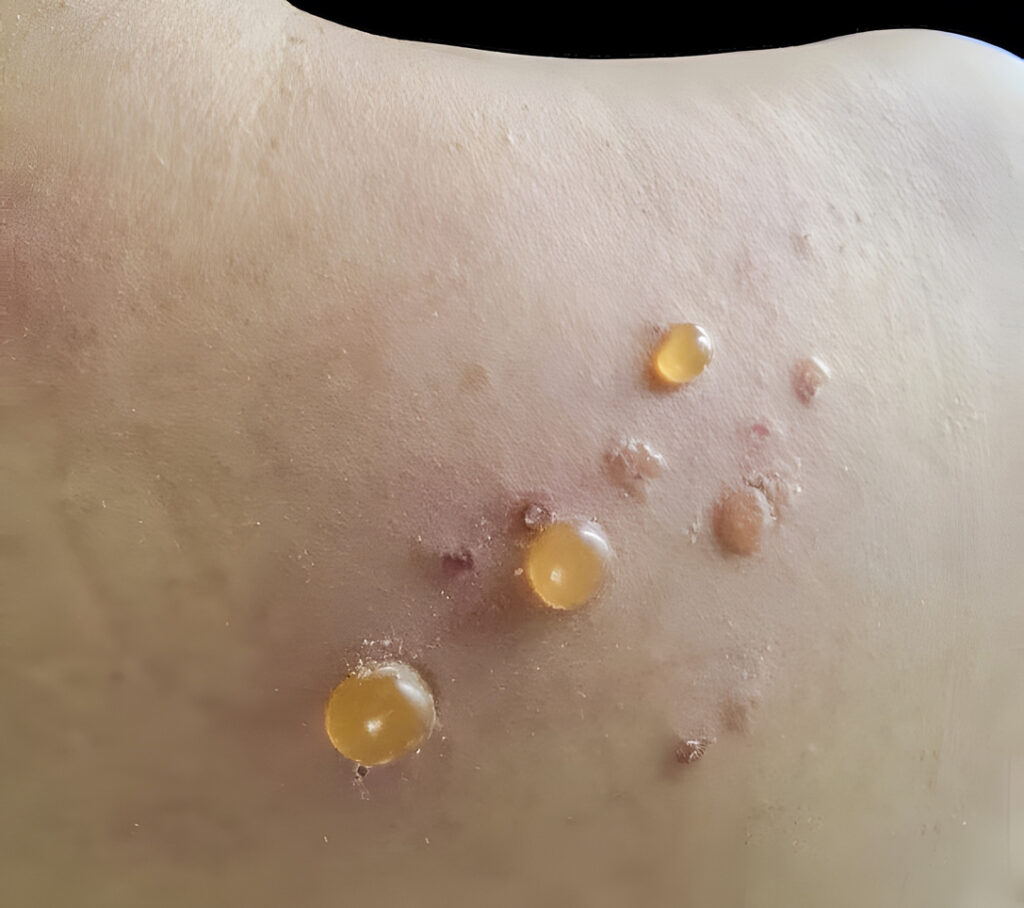
The U.S. Food and Drug Administration (FDA) has accepted the supplemental biologics license application (sBLA) for dupilumab (Dupixent, Sanofi & Regeneron) to treat adults with bullous pemphigoid (BP) for priority review.
If approved, dupilumab would be the first and only targeted medicine to treat BP in the US. The FDA decision is expected by June 20, 2025.
Priority review is granted to regulatory applications seeking approval for therapies that have the potential to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. Dupilumab was previously granted orphan drug designation by the FDA for BP, which applies to investigational medicines intended for the treatment of rare diseases that affect fewer than 200,000 people in the US.
The sBLA is supported by data from a pivotal study evaluating the efficacy and safety of dupilumab in 106 adults with moderate-to-severe BP. The primary endpoint was met, with five times more dupilumab patients achieving sustained disease remission compared to those on placebo. Sustained disease remission was defined as complete clinical remission with completion of oral corticosteroids (OCS) taper by week 16 (off OCS treatment and only treated with dupilumab for at least 20 weeks) without relapse and no rescue therapy use during the 36-week treatment period. The study also showed that dupilumab significantly reduced disease severity, itch, and use of OCS compared to placebo.
Adverse events more commonly observed with dupilumab (in at least three patients) compared to placebo included peripheral edema, arthralgia, back pain, blurred vision, hypertension, asthma, conjunctivitis, constipation, upper respiratory tract infection, limb injury, and insomnia.
The safety and efficacy of Dupixent in BP are currently under clinical assessment and have not been evaluated by any regulatory authority.