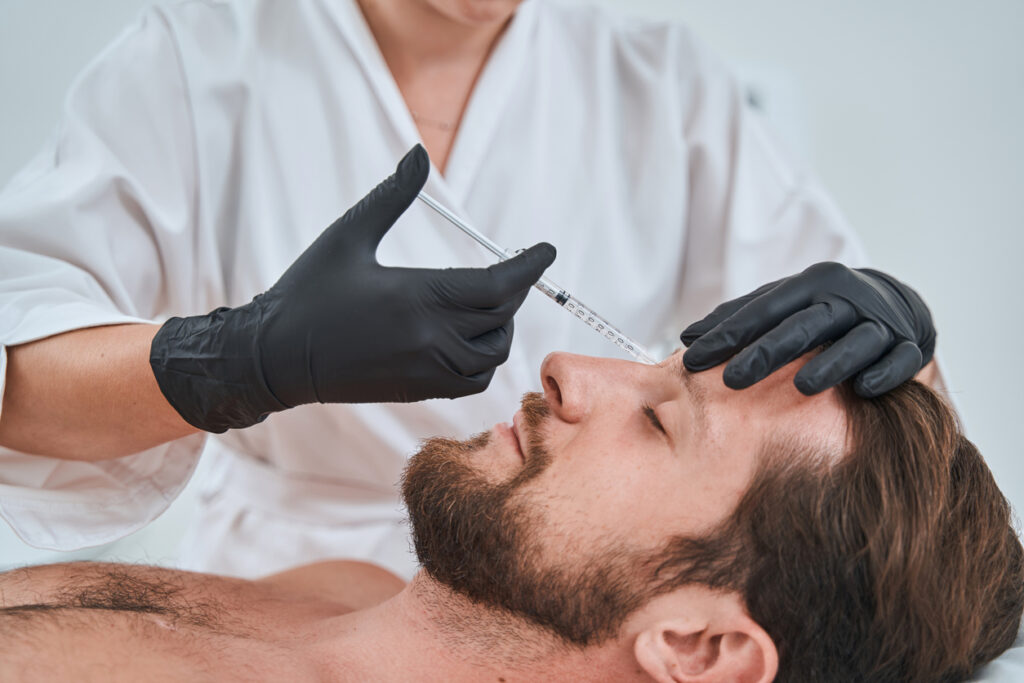
Evolus, Inc. is rolling out Nuceiva (botulinum toxin type A) across Spain.
The product is now available for direct order and delivery to Spanish medical aesthetics healthcare professionals.
Nuceiva is approved by the European Commission for the temporary improvement in the appearance of moderate to severe glabellar lines when the severity of the above facial lines has an important psychological impact in adults below 65 years of age. Nuceiva is licensed in the United States under the brand name Jeuveau.
The safety and efficacy of Nuceiva was evaluated through the company’s TRANSPARENCY clinical program – three Phase III trials including the largest head-to-head aesthetic pivotal study versus Botox (onabotulinumtoxinA) to date, and two long-term safety studies. Side effects were similar to others in this class of medicine.