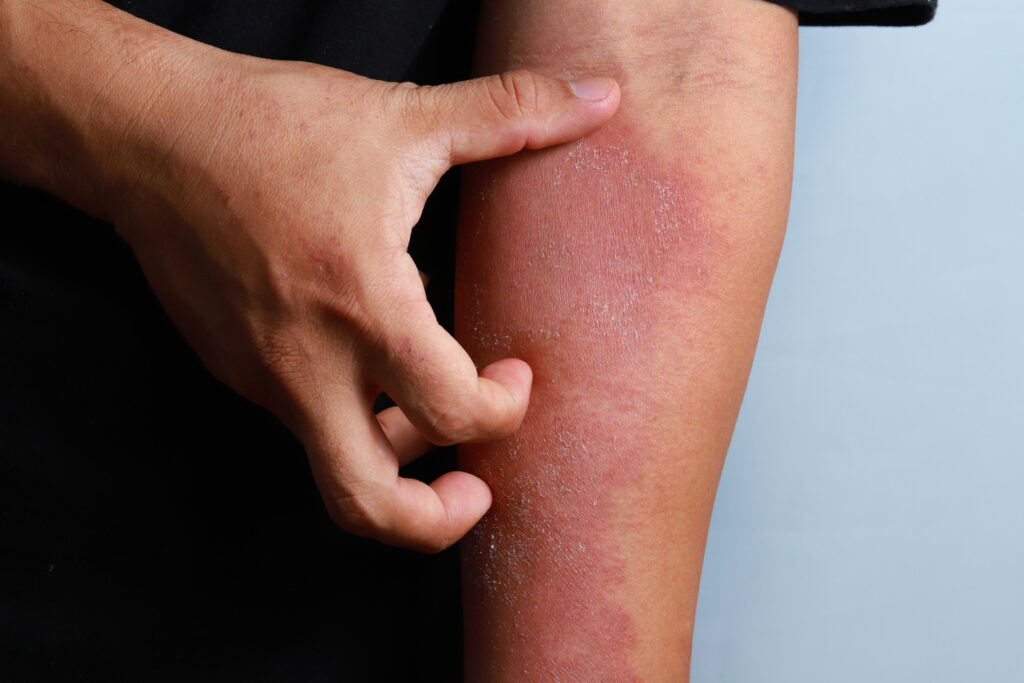
Rocatinlimab, an investigational therapy targeting the OX40 receptor, met its co-primary endpoints in a study of adults with moderate to severe atopic dermatitis (AD), according to top-line data from the Phase 3 ROCKET HORIZON Trial released at the European Academy of Dermatology and Venereology (EADV) Congress in Amsterdam.
Co-primary endpoints in this study included achievement of a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-ADTM) score of 0 (clear) or 1 (almost clear) with a ≥ 2-point reduction from baseline and achievement of ≥ 75% reduction from baseline in Eczema Area and Severity Index score (EASI-75).
Fully 19.3% patients who received rocatinlimab hit vIGA-ADTM of 0 or 1 compared to 6.6% of people who took placebo at Week 24, and 32.8% of patients on rocatinlimab hits EASI-75 by Week 24, compared with 13.7% of people in the placebo arm.
The trial also met the revised Investigator Global Assessment (rIGA 0/1), a more stringent measure of efficacy than vIGA 0/1 based on a narrower definition of 1 (almost clear), at week 24, with 16.4% of people in the rocatinlimab arm hitting this mark, compared with 4.9% of those taking placebo.
The study also reached statistically significant differences from placebo for all key secondary endpoints, which include measurements of skin clearance (vIGA 0/1 and EASI-75 at week 16 and EASI-90 at week 24), the Pruritus Numeric Rating Scale, Atopic Dermatitis Skin Pain Scale, Dermatology Quality of Life Index, and severity scores of hand atopic dermatitis and facial atopic dermatitis. Overall safety findings in ROCKET HORIZON were comparable to those seen in the Phase 2b study.
“We are very pleased to have achieved statistically significant efficacy results over placebo which are consistent for co-primary endpoints and all key secondary endpoints. We look forward to further demonstrating that rocatinlimab, a potential T-cell rebalancing therapy, may help patients with moderate to severe atopic dermatitis as a new therapeutic option. We anticipate getting additional data from the ROCKET program and fully understanding the value rocatinlimab can deliver to patients.” says Takeyoshi Yamashita, PhD, senior managing executive officer and chief medical officer at Kyowa Kirin, in a news release.
Detailed results of HORIZON will be provided at a future medical congress. HORIZON is one of eight studies in the global ROCKET Phase 3 clinical trial program. Amgen and Kyowa Kirin plan to review HORIZON and forthcoming results from the other seven studies in the ROCKET program as part of ongoing discussions with global regulatory authorities.