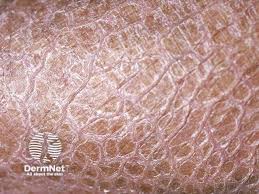
TMB-001 did not meet the primary and key secondary endpoints in a randomized, double-blind period of the phase 3 ASCEND clinical trial of congenital ichthyosis.
The trial results do not support submission of a New Drug Application to the U.S. Food and Drug Administration, according to Leo Pharma and Timber Pharmaceuticals.
The trial did not show a statistically significant difference between the proportion of patients treated with TMB-001 responding to treatment after 12 weeks compared with patients treated with vehicle.
ASCEND is the Phase 3 clinical trial of TMB-001, an investigational topical ointment formulation of isotretinoin for the potential treatment of patients with moderate to severe congenital ichthyosis. The trial evaluated the efficacy and safety of TMB-001 in the treatment of congenital ichthyosis in patients (aged 6 or older) with either the autosomal recessive congenital ichthyosis (ARCI) or X-linked recessive ichthyosis (XLRI) subtypes.
The majority of adverse events in ASCEND were non-serious localized skin reactions of mild or moderate severity. Detailed results from ASCEND are planned to be submitted for scientific publication at a later date.
“We are disappointed and saddened by the results of the phase 3 trial. After encouraging phase 2b results, we observed an unexpectedly high vehicle response in this trial. We had hoped that TMB-001 could have been a new potential treatment to help children and adults suffering from moderate to severe congenital ichthyosis and to allow them to live a quality life they had previously not known,” says John Koconis, Chief Executive Officer of Timber Pharmaceuticals, in a news release.
LEO Pharma acquired TMB-001 from Timber Pharmaceuticals in January 2024 following Timber’s Chapter 11 bankruptcy filing. Timber Pharmaceuticals was a clinical-stage biopharmaceutical rare disease dermatology company focused on the development of treatments for rare and orphan dermatologic diseases, and TMB-001 had received orphan, fast-track, and break-through designation by the FDA. As a consequence of the acquisition, Timber Pharmaceuticals was reformed as a fully owned subsidiary of LEO Pharma.
PHOTO CREDIT: DermNet