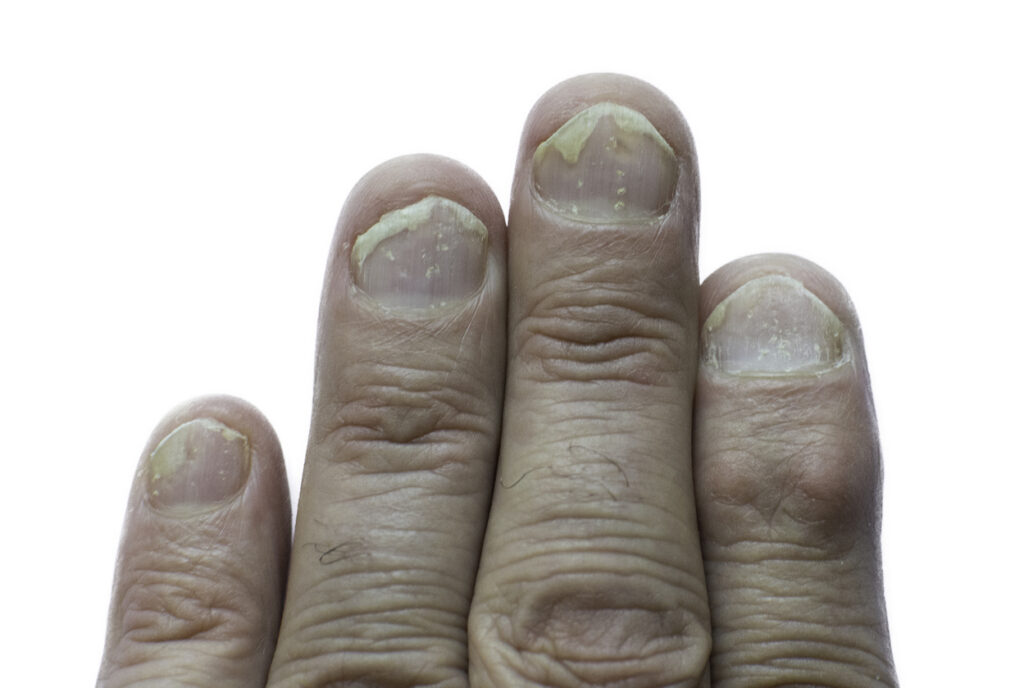
Tildrakizumab-asmn (Ilumya, Sun Pharmaceutical Industries, Inc.) improves moderate-to-severe nail psoriasis, according to new research presented at the 2025 American Academy of Dermatology (AAD) Annual Meeting in Orlando, FL.
The Phase 3b, randomized, double-blind, placebo-controlled clinical trial included 99 patients with moderate-to-severe plaque psoriasis. Patients treated with tildrakizumab demonstrated a significantly higher rate of 75% improvement from baseline in the Nail Psoriasis Severity Index (mNAPSI 75) at Week 28 (25.5% mNAPSI 75 response rate vs. 4.5% in the placebo group.
In addition, 29.4% of patients treated with tildrakizumab achieved normal nails or nails with minimal psoriasis (ViSENPsO score of 0 or 1), compared to 4.2% in the placebo group. (ViSENPsO is a clinical tool used for detecting and assessing psoriasis including a visual medical scale to evaluate nail psoriasis severity and Patient Global Assessment (PGA) questionnaires.)
“Nail psoriasis is understood as a difficult to treat population, and many current therapies fall short of providing lasting relief or are slow to produce results,” says study author Paul Yamauchi, MD, a Dermatologist in Santa Monica, CA, in a news release. “While other IL-23 inhibitors do not have a dedicated study to evaluate efficacy in nail psoriasis, these trial results clearly demonstrate the promise for ILUMYA to ease discomfort and improve patient outcomes.”
The safety profile was consistent with the known safety profile of tildrakizumab, and no serious adverse events related to ILUMYA treatment occurred in this clinical trial,the study showed. The most common (≥1%) adverse reactions associated with tildrakizumab treatment that were more frequent than in the placebo group are upper respiratory infections, injection-site reactions, and diarrhea.
“Psoriasis affecting the nails can have a profound impact on a patient’s daily life, causing both physical discomfort and emotional distress. Although highly prevalent in people with plaque psoriasis, nail psoriasis has been challenging to treat,” adds Marek Honczarenko, MD, PhD, Senior Vice President, Head of Development, Sun Pharma, North America. “As a company dedicated to developing innovative therapies which help improve quality of life for patients, these data, along with our existing data in scalp psoriasis, reinforces the benefits of ILUMYA in difficult to treat populations.”