By Irene Li Quan and Peter Lio, MD
Introduction
Atopic dermatitis (AD), or eczema, manifests as a chronic, itchy rash with a large impact on quality of life. It is one of the most common and burdensome skin diseases worldwide.1 While AD occurs in all age groups, it affects up to 20% of children globally and can both present in and persist into adulthood.2 Despite its importance, there is a substantial knowledge gap regarding the relationship between AD and climate change. Climatic conditions play a pivotal role in the pathophysiology of AD, impacting skin physiology through mechanisms such as skin barrier impairment, immune dysregulation, cutaneous dysbiosis, and chronic pruritus.3 Key climatic hazards, namely ultraviolet (UV) radiation, air pollution, and erratic temperatures and humidity, may exacerbate AD in individuals who are especially susceptible to climatic factors. These environmental factors have incredibly complex interactions with the pathways of AD pathogenesis. For instance, toxic levels of UV radiation can induce apoptosis and reduce the expression of key proteins like filaggrin and E-cadherin in the skin barrier.4 In the immune system, air pollution and other irritants contribute to inflammation via aryl hydrocarbon receptor (AhR) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB).5 Finally, rising temperatures and resultant sweating can intensify pruritus, contributing to AD’s itch-scratch cycle.6
These mechanisms highlight the need for further research to shed light on long-term effects, particularly in vulnerable populations. While there may be ongoing debate about the human contribution to climate change, the consensus is clear that the climate is indeed changing. This will likely have many implications for people all over the world, and they may be different in different areas. We review some of the possible ramifications of AD, knowing that predicting the future is fraught with difficulty. In this brief narrative review, we focus on the major climatic hazards, specifically air pollution and rising temperatures, and how their adverse effects on AD and skin health are exacerbated based on geographic region and among at-risk populations.
Climatic Hazards
Air pollution and wildfires
As climate change progresses, the prevalence of pollutants from wildfires and air pollution is concurrently rising. Importantly, exposure to these pollutants causes cumulative damage to the skin. Recent wildfires in Canada and in Maui, HI, caused elevated pollution levels and dire health impacts that spread across the U.S., leading to record-high air pollution levels in New York City in 2023 and some of the worst wildfires in Maui in over a century.7 Evidently, wildfires are one of the biggest generators of air pollution, with up to 90% of inhalable particle mass consisting of particulate matter that is 2.5 micrometers in diameter or smaller. These particles are referred to as PM2.5 and are small enough to penetrate the lungs and even enter the bloodstream, posing significant health risks.
Both wildfires and air pollutants exacerbate chronic skin conditions and trigger acute flare-ups. The biological mechanisms underlying the effects of wildfire smoke and air pollutants weaken the skin barrier over time, making it vulnerable to inflammation and continued damage. For instance, particulate matter and cigarette smoke disrupt the skin barrier, induce oxidative stress, promote proinflammatory signals, and cause dysbiosis.4 Further exposure to toxins like PM2.5 and carbon monoxide is associated with premature aging and an increased disease burden for individuals with pre-existing skin conditions.8
Exposure to isocyanates
Another environmental factor related to the pathogenesis of AD is exposure to isocyanates. Studies have shown that isocyanates are thought to cause skin dysbiosis through microbial adaptations to modern pollutants. Zeldin et al. proposed that exposure to isocyanates, especially diisocyanates, correlates with the historical increase in AD prevalence, which they attribute to metabolic changes in disease-associated microbes.9 While isocyanate exposure is linked to some natural causes of AD, like wildfire smoke, there is an increasing amount of evidence connecting AD to chemical triggers in the modern era. Some epidemiological risk factors related to isocyanate and microbe exposure include smoke from factories, high-octane gasoline from cars and planes, oil refineries, and toxic household products like synthetic paints, adhesives, and textiles.10 (See Table 1.)
The biological mechanism of how isocyanates induce skin dysbiosis is through the host itch receptor TH2-responsive thermoreceptor (TRPA1) and the inhibition of ceramide production.11 Pollution disrupts the normal metabolic pathways of commensal microbiota, subsequently disturbing the symbiotic interactions between health-promoting and disease-promoting organisms. For example, the metabolism of Roseomonas mucosa depends on the production of glycolipids, which create ceramides that protect the skin.10 Exposure to isocyanates inhibits the production of these lipids and thereby negatively affects the barrier, leading to overgrowth of the pathogenic Staphylococcus aureus. (See Figure 1.)
Other sources of isocyanates linked to AD and skin dysbiosis include benzene, toluene, ethylbenzene, and, most significantly, xylene, collectively referred to as “BTEX compounds.” The study by Ratley et al. used spatial modeling to demonstrate the geographic association between isocyanates in air pollutants and other modern toxins with AD. By analyzing Environmental Protection Agency databases, pollution data, and clinical visits, the authors concluded that BTEX compounds, particularly xylene, led to more environmentally-induced AD flares and are one of the most important predictors of AD. High odds ratios were found for synthetic or isocyanate-containing textiles (e.g., polyester, nylon, spandex), along with associated shifts away from the therapeutic pathways of health-associated R. mucosa and S. epidermidis.12
Unpredictable temperature changes
Climate change has resulted in unpredictable temperature variations. An increasing body of literature has been published on the diverse effects of hot and cold temperatures on the development of AD, particularly in relation to skin barrier dysfunction, itching, skin flare-ups, and other allergic reactions such as asthma exacerbations.
Increased temperatures trigger proinflammatory cytokine production and pruritus through transient receptor potential vanilloid (TRPV) channels 1, 3, and 4.13 A study in the U.S. found that higher temperatures and sun exposure over eight years correlated with increased AD severity in children.14 Conflictingly, however, a study in Italy showed that increased temperature and humidity were linked to decreased AD severity.15 Many other studies have shown an association between higher temperatures, heat stress, and increased morbidity and mortality.16 This variation suggests that the effects of warming on AD may depend on baseline temperature, geography, and other climatic factors.
Both heat and sweating can trigger an itching sensation in AD patients. Studies conducted indoors and outdoors have resulted in AD exacerbations. One case-control study specifically reported a correlation between the use of radiators in children’s bedrooms and AD prevalence.17 Though it is still being researched, the pathogenesis of itch in AD is thought to be linked to thermosensitive TRP channels, including TRPV1, TRPV3, and TRPV4. Studies in mouse models and clinical trials have shown that TRPV1 antagonists, such as PAC-14028, successfully alleviate AD-like symptoms by inhibiting capsaicin-induced Ca2+ influx and blood perfusion in the skin.18 Additionally, TRPV3 antagonists can reduce heat induced by thymic stromal lymphopoietin (TSLP), a key factor in the “atopic march” that promotes skin and systemic allergic inflammation, and are currently being explored to control pruritus.19 TRPV4 inhibitors specifically target skin damage, pain, and itching caused by sunburn and UV exposure.20
Moreover, high temperatures and subsequent sweating may contribute to subclinical miliaria and induce AD. Indirect effects of global warming also lead to the widespread dispersion of aeroallergens and allergen proteins, intensified pollen seasons, and exacerbated allergic flares in patients with AD.21
Conversely, there are several mechanisms through which cold temperatures can affect the skin barrier and trigger AD. Compared to the pathways through which high temperatures affect the skin, cold temperatures primarily act via TRPV1 receptors, which are expressed on epithelial cells and nociceptive afferent fibers and regulate the influx of cations.22 TRPV1 downregulates filaggrin and loricrin, resulting in pruritus and skin barrier dysfunction.13 Lower temperatures have also been reported to activate proinflammatory cytokines like IL-1β, TSLP, and TNF-alpha, ultimately disrupting protein and skin barrier function.13
Several studies have highlighted the skin’s response to lower outdoor temperatures. The International Study of Asthma and Allergies in Childhood (ISAAC) was conducted in Spain and Mexico and found that the prevalence of “an itchy rash” in areas such as the elbows, knees, ankles, buttocks, neck, ears, and eyes increased with lower mean outdoor temperatures.23 The ISAAC study also reported that temperature drops affect skin hydration and water loss. Reduced moisture levels weaken the skin barrier, making it more susceptible to damage from skin irritants, allergens, and mechanical stressors.24
A meta-analysis of studies from several European and Asian countries in the Northern Hemisphere revealed that children born in the fall and winter had a higher incidence of AD compared to those born in the spring and summer.25 These findings were corroborated by a birth cohort study and a registry study in Denmark.26 In Asia, a questionnaire-based study in Korea showed the highest AD prevalence in winter, while two studies in Japan indicated the highest prevalence in fall.27,28 In the U.S., the highest AD prevalence in children was observed in fall, based on analyses of Medicaid claims data and electronic medical record diagnoses at birth.29
Temperature and climate are also thought to influence the perception of AD symptom severity, itching, and flares through seasonal changes. Agner et al. demonstrated that the epidermis is less hydrated in the winter, exacerbating the skin’s response to irritants. Similarly, Langan et al. found that winter weather induced more shampoo-associated skin flares, and Vocks et al. elucidated an inverse relationship between temperature and itch intensity.30, 31
Overall Impact
Effect of geography
The impact of climatic hazards, such as wildfire smoke, air pollution, and global warming, on AD varies depending on geographic region. These hazards disproportionately affect vulnerable populations, including underserved communities, the elderly, and pediatric groups, both within countries and across borders.32
Research conducted in the U.S. reveals significantly higher indoor air pollution levels in households near roadways or lacking air conditioning, particularly among those who frequently leave their windows open.33 This regional pollution disparity raises additional concerns for the uneven distribution of AD onset and exacerbations.
On a global scale, studies conducted in different countries demonstrate varying effects of temperature on AD symptoms. For instance, higher temperatures in southern Italy and Denmark are correlated with reduced AD severity and healthcare utilization, respectively.15, 34 Conversely, warmer weather in the U.S. is associated with more unpredictable symptoms in children with AD.14 These variations highlight the need for more comprehensive studies to be conducted to isolate the specific effects of climatic factors on AD prevalence while accounting for other population risk factors.
Effect on vulnerable populations
Climatic hazards heighten the risk and severity of AD not only regionally and nationally, but also internationally due to climate-induced migration. The United Nations High Commissioner for Refugees recognizes refugees, migrants, asylum seekers, and internally displaced people as the “frontlines of the climate emergency.”35 Among refugees, AD prevalence varies widely, from 10.5% in rural Sudan to 33% among Syrians in Jordan.36, 37 AD is exacerbated by the poor environmental conditions of refugee camps and immigrant communities, characterized by mold, pests, overcrowding, poor ventilation, and elevated infection risks.38
Indirectly, the climate crisis affects psychosocial stress, which is notably higher in displaced populations compared to the general population. Research indicates that conditions such as depression, anxiety, and post-traumatic stress disorder contribute to a higher prevalence of itch and inflammatory mediator release in patients with AD.39
Current Initiatives
Given the geographic disparities in environmental exposure across communities, prioritizing effective environmental policies and resources is crucial to safeguard skin health and overall well-being, particularly for at-risk populations. Several countries have implemented successful interventions to combat air pollution. In the U.S., the Clean Air Act of 1990 was a landmark legislation that significantly reduced air pollutant concentrations.40 Similarly, countries like China, Poland, and the Netherlands have improved air quality by deploying air purifier towers and implementing smog-reduction strategies.41
Current initiatives targeting wildfires and air pollution emphasize community-based mitigation efforts. In response to recent wildfires, the Centers for Disease Control and Prevention issued recommendations for wearing N95 masks, using portable air filters, and establishing communal “clean air centers” in public spaces such as schools and libraries.42 In 2023, Congress introduced the Cleaner Air Spaces Act, which includes provisions to establish these centers, provide free air filtration units, and support community-based environmental advocacy organizations.43 These measures not only support the needs of individuals and households at high risk of smoke exposure and air pollution, but also reduce concentrations of PM2.5, CO, and other volatile organic compounds, hopefully preventing exacerbations of AD.
Conclusion
While government and community organizations have made efforts to advance environmental policies aimed at reducing disparate exposures to air pollution, much remains to be uncovered about the relationship between the environment, AD, and overall skin health. The current knowledge base is still limited to a few climatic hazards and is complicated by varying effects at the population and climatic levels. Future research should explore a comprehensive range of climatic factors and events, monitor longitudinal disease activity of AD at the individual level, and consider differential impacts on vulnerable subpopulations. The accumulation of information on phenomena such as microbiota pathways and BTEX-induced dysbiosis can inform policies aimed at reducing the production of harmful household products and promote the development of filtration systems against air pollutants.
Barry Commoner, a leading ecologist and one of the founders of the modern environmental movement, once said, “Environmental pollution is an incurable disease. It can only be prevented.”44 The United Nations’ Environment Programme took this sentiment even further in 2022, declaring that we must now learn how to coexist with—rather than fight—the wildfires around us.45 It is imperative for healthcare practitioners, government agencies, and community organizations to collaborate in preserving the well-being of patients and preventing exacerbations of chronic diseases. Dermatologists, in particular, can play an active role in advocating for increased patient and medical education on the dermatologic effects of air pollution and supporting policies that prioritize the health of all patients.
Table 1. Summary of Studies and Outcomes
| Title | Authors (Year) | Climatic Hazard | Mechanism | Effects on AD | Country |
| The burden of air pollution on skin health: a brief report and call to action
|
Santiago Mangual KP, Ferree S, Murase JE, Kourosh AS (2024) | Wildfires and CO levels | Activate oxidative stress and inflammation, disrupting skin barrier and skewing immune response towards Th2 phenotype | Increased AD dermatology clinic visits | USA |
| Skin damage mechanisms related to airborne particulate matter exposure | Huang CH, Chen SC, Wang YC, Wang CF, Hung CH, Lee SS. (2016) | Airborne particulate matter (PM) | Disrupt skin barrier, inducing oxidative stress and inflammation | Increased AD incidence and exacerbation | Argentina |
| Exposure to isocyanates predicts atopic dermatitis prevalence and disrupts therapeutic pathways in commensal bacteria | Zeldin J, Chaudhary PP, Spathies J, et al. (2023) | Isocyanates | Disrupt production of beneficial lipids to cause skin dysbiosis through microbial adaptations to modern pollutants | Increased AD diagnosis rates | USA |
| Spatial modeling connecting childhood atopic dermatitis prevalence with household exposure to pollutants | Ratley G, Zeldin J, Sun AA, Yadav M, Chaudhary PP, Myles IA (2024) | BTEX compounds | Activate TRPA1 to cause skin dysbiosis | Increased environmentally induced AD flares | USA |
| Warm, humid, and high sun
exposure climates are associated with poorly controlled eczema: PEER (pediatric eczema elective registry) cohort, 2004-2012 |
Sargen MR, Hoffstad O, Margolis DJ. (2024) | Increased temperatures | Increased heat and humidity lead to increased sweating, furthering an irritant effect, Th2 and Th17-mediated inflammation, water evaporation on the skin | Poorly controlled AD | USA |
| Atopic dermatitis severity during exposure to air pollutants and weather changes with an Artificial Neural Network (ANN) analysis | Patella V, Florio G, Palmieri M, et al. (2020) | Increased outdoor temperatures | Induce oxidative stress, leading to skin barrier dysfunction or immune response dysregulation | Increased AD severity | Italy |
| The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods | Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart AW, ISAAC Steering Committee (2008) | Decreased temperatures | Humidity increases the number of mast cells in the dermis | Increased prevalence of AD symptoms | Spain, Mexico |
| Neonatal risk factors of atopic dermatitis in Denmark: results from a nationwide register-based study | Egeberg A, Andersen YMF, Gislason G, Skov L, Thyssen JP. (2016) | Seasonality | Vitamin D deficiency | Increased AD incidence in children born in the fall and winter | Denmark |
| The effect of season of birth on atopic dermatitis and food allergy | Dunlop JH, Keller JP, Peng RD, Keet CA | Seasonality | Vitamin D deficiency | Increased AD incidence in children born in the fall | USA |
| Environmental factors associated with childhood eczema: findings from a national web-based survey | Sasaki M, Yoshida K, Adachi Y, et al (2016) | Seasonality | Vitamin D deficiency | Increased AD incidence in children born in the fall | Japan |
| The relationships among birth season, sunlight exposure during infancy, and allergic disease | Hwang JM, Oh SH, Shin MY (2016) | Seasonality | Insufficient sunlight exposure and vitamin D deficiency | Increased AD incidence in children born in the winter | Korea |
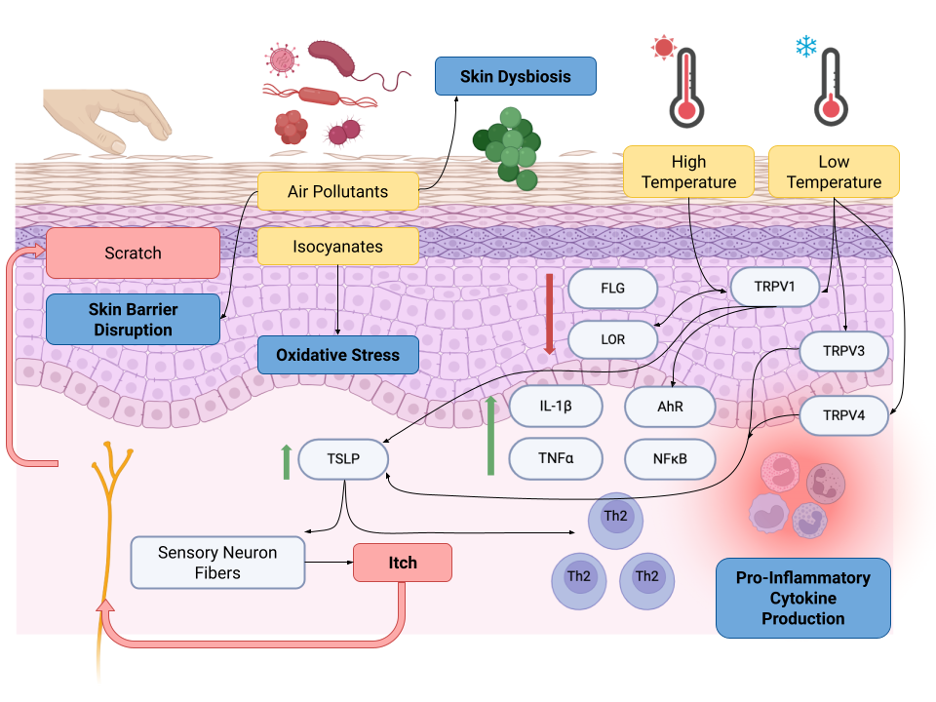
Figure 1.
Mechanisms of the impact of climatic hazards on the skin physiology of AD. Climatic hazards (as depicted by the yellow boxes in the diagram) include air pollutants and isocyanates, high temperature and low temperature. Climate change increases the prevalence, severity, and exacerbations of AD through skin barrier disruption, oxidative stress, pro-inflammatory cytokine disruption, and skin dysbiosis (as depicted by the bolded blue boxes in the diagram). Heat, humidity, and sweating drive chronic pruritus and the itch-scratch cycle that is characteristic of AD. FLG, filaggrin; LOR, loricrin; TRPV, transient receptor potential vanilloid; IL, interleukin; TNF, tumor necrosis factor; AhR, aryl hydrocarbon receptor; NFκB, nuclear factor kappa B; TSLP, thymic stromal lymphopoietin. (Created with BioRender.com.)
References:
References:
1.Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017*. Br J Dermatol. 2021;184(2):304-309.
2.Oykhman P, Dookie J, Al-Rammahy H, et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2022;10(10):2657-2666.e8.
3. Wang SP, Stefanovic N, Orfali RL, et al. Impact of climate change on atopic dermatitis: a review by the International Eczema Council. Allergy. Published online January 24, 2024. doi:10.1111/all.16007
4.Hendricks AJ, Eichenfield LF, Shi VY. The impact of airborne pollution on atopic dermatitis: a literature review. Br J Dermatol. 2020;183(1):16-23.
5. Magnani ND, Muresan XM, Belmonte G, et al. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol Sci. 2016;149(1):227-236.
6.Hui-Beckman JW, Goleva E, Leung DYM, et al. The impact of temperature on the skin barrier and atopic dermatitis. Ann Allergy Asthma Immunol. 2023;131(6):713-719.
7. Santiago Mangual KP, Ferree S, Murase JE, et al. The bbrden of air pollution on skin health: a brief report and call to action. Dermatol Ther. 2024;14(1):251-259.
8. Huang CH, Chen SC, Wang YC, et al. Detrimental correlation between air pollution with skin aging in Taiwan population. Medicine. 2022;101(31):e29380.
9. Zeldin J, Chaudhary PP, Spathies J, et al. Exposure to isocyanates predicts atopic dermatitis prevalence and disrupts therapeutic pathways in commensal bacteria. Sci Adv. 2023;9(1):eade8898.
10. Yadav M, Chaudhary PP, D’Souza BN, et al. Diisocyanates influence models of atopic dermatitis through direct activation of TRPA1. PLoS One. 2023;18(3):e0282569.
11. Mihara S, Shibamoto T. The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin Immunol. 2015;11(1):11.
12. Ratley G, Zeldin J, Sun AA, et al. Spatial modeling connecting childhood atopic dermatitis prevalence with household exposure to pollutants. Commun Med. 2024;4(1):74.
13. Kim BE, Hui-Beckman J, Lyubchenko T, et al. Transient receptor potential vanilloid 1 plays a major role in low temperature-mediated skin barrier dysfunction. J Allergy Clin Immunol. 2022;150(2):362-372.e7.
14. Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134(1):51-57.
15. Patella V, Florio G, Palmieri M, et al. Atopic dermatitis severity during exposure to air pollutants and weather changes with an Artificial Neural Network (ANN) analysis. Pediatr Allergy Immunol. 2020;31(8):938-945.
16. Costello A, Romanello M, Hartinger S, et al. Climate change threatens our health and survival within decades. Lancet. 2023;401(10371):85-87.
17. McNally NJ, Williams HC, Phillips DR. Atopic eczema and the home environment. Br J Dermatol. 2001;145(5):730-736.
18. Yun JW, Seo JA, Jeong YS, et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J Dermatol Sci. 2011;62(1):8-15.
19. Seo SH, Kim S, Kim SE, et al. Enhanced thermal sensitivity of TRPV3 in keratinocytes underlies heat-induced pruritogen release and pruritus in atopic dermatitis. J Invest Dermatol. 2020;140(11):2199-2209.e6.
20. Moore C, Cevikbas F, Pasolli HA, et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A. 2013;110(34):E3225-E3234.
21. Fölster-Holst R, Galecka J, Weißmantel S, et al. Birch pollen influence the severity of atopic eczema – prospective clinical cohort pilot study and ex vivo penetration study. Clin Cosmet Investig Dermatol. 2015;8:539-548.
22. Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10(8):601-620.
23. Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart AW, ISAAC Steering Committee. The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods. Int J Tuberc Lung Dis. 2005;9(1):10-16.
24. Engebretsen KA, Johansen JD, Kezic S, et al. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30(2):223-249.
25. Calov M, Alinaghi F, Hamann CR, et al. The association between season of birth and atopic dermatitis in the northern hemisphere: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2020;8(2):674-680.e5.
26. Egeberg A, Andersen YMF, Gislason G, et al. Neonatal risk factors of atopic dermatitis in Denmark: results from a nationwide register-based study. Pediatr Allergy Immunol. 2016;27(4):368-374.
27. Hwang JM, Oh SH, Shin MY. The relationships among birth season, sunlight exposure during infancy, and allergic disease. Korean J Pediatr. 2016;59(5):218-225.
28. Sasaki M, Yoshida K, Adachi Y, et al. Environmental factors associated with childhood eczema: findings from a national web-based survey. Allergol Int. 2016;65(4):420-424.
29. Dunlop JH, Keller JP, Peng RD, et al. The effect of season of birth on atopic dermatitis and food allergy. Ann Allergy Asthma Immunol. 2020;125(2):221-223.e2.
30. Agner T, Serup J. Seasonal variation of skin resistance to irritants. Br J Dermatol. 1989;121(3):323-328.
31. Vocks E, Busch R, Fröhlich C, et al. Influence of weather and climate on subjective symptom intensity in atopic eczema. Int J Biometeorol. 2001;45(1):27-33.
32. Hajat A, Hsia C, O’Neill MS. Socioeconomic Disparities and Air Pollution Exposure: a global review. Curr Environ Health Rep. 2015;2(4):440-450.
33. Shrestha PM, Humphrey JL, Carlton EJ, et al. Impact of outdoor air pollution on indoor air quality in low-income homes during wildfire seasons. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193535
34. Hamann CR, Andersen YMF, Engebretsen KA, et al. The effects of season and weather on healthcare utilization among patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2018;32(10):1745-1753.
35. Climate change and disaster displacement. UNHCR US. Accessed May 1, 2024. https://www.unhcr.org/en-us/climate-change-and-disasters.html
36. Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58(11):1341-1349.
37. Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34(2):419-425.
38. Habib RR, Basma SH, Yeretzian JS. Harboring illnesses: on the association between disease and living conditions in a Palestinian refugee camp in Lebanon. Int J Environ Health Res. 2006;16(2):99-111.
39. Hashizume H, Horibe T, Ohshima A, et al. Anxiety accelerates T‐helper 2‐tilted immune responses in patients with atopic dermatitis. Br J Dermatol. 2005;152(6):1161-1164.
40. Greenbaum DS. The Clean Air Act: substantial success and the challenges ahead. Ann Am Thorac Soc. 2018;15(3):296-297.
41. Burki TK. The innovations cleaning our air. Lancet Respir Med. 2019;7(2):111-112.
42. Stone SL, Anderko L, Berger MF, et al. Wildfire smoke: a guide for public health officials. U.S. Environmental Protection Agency. Published online 2019. https://apha.confex.com/apha/2017/meetingapi.cgi/Paper/386395?filename=2017_Abstract386395.html&template=Word
43. Peters SH. Cleaner Air Spaces Act of 2023. 2023. Accessed May 3, 2024. https://www.congress.gov/bill/118th-congress/house-bill/4077/text
44. Vinciguerra T. At 90, an environmentalist from the ’70s still has hope. The New York Times. Published June 19, 2007. Accessed May 5, 2024. https://www.nytimes.com/2007/06/19/science/earth/19conv.html
45. Ambassadors G. As climate changes, world grapples with a wildfire crisis. UNEP. Published March 9, 2022. Accessed May 5, 2024. https://www.unep.org/news-and-stories/story/climate-changes-world-grapples-wildfire
Irene Quan
Irene Quan is a second-year MD/MPH candidate at Northwestern University Feinberg School of Medicine in Chicago, IL.
DISCLOSURES: None
Peter A. Lio, MD
Peter A. Lio, MD, is a Clinical Assistant Professor of Dermatology and Pediatrics at Northwestern University Feinberg School of Medicine and a partner at Medical Dermatology Associates of Chicago in Chicago, IL.
DISCLOSURES:
Dr. Lio reports research grants/funding from AbbVie, AOBiome, Eczema Foundation, National Eczema Association; is on the speaker’s bureau for AbbVie, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oreal, MyOR Diagnostics, ParentMD, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme; reports consulting/advisory boards for AbbVie, Almirall, Amyris, Arbonne, Arcutis, ASLAN, Boston Skin Science, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs, Concerto Biosci, Dermavant, DermVeda, Eli Lilly, Galderma, IntraDerm, Janssen, Johnson & Johnson, Kaleido Biosci, Kimberly Clark, LEO Pharma, Lipidor, L’Oreal, Menlo Therapeutics, Merck, Micreos, MyOR Diagnostics, Regeneron/Sanofi Genzyme, Skinfix, Sonica, Theraplex, UCB, Unilever, Verrica, Yobee Care; stock options: Micreos, Modernizing Medicine, Yobee Care. In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member of the National Eczema Association.