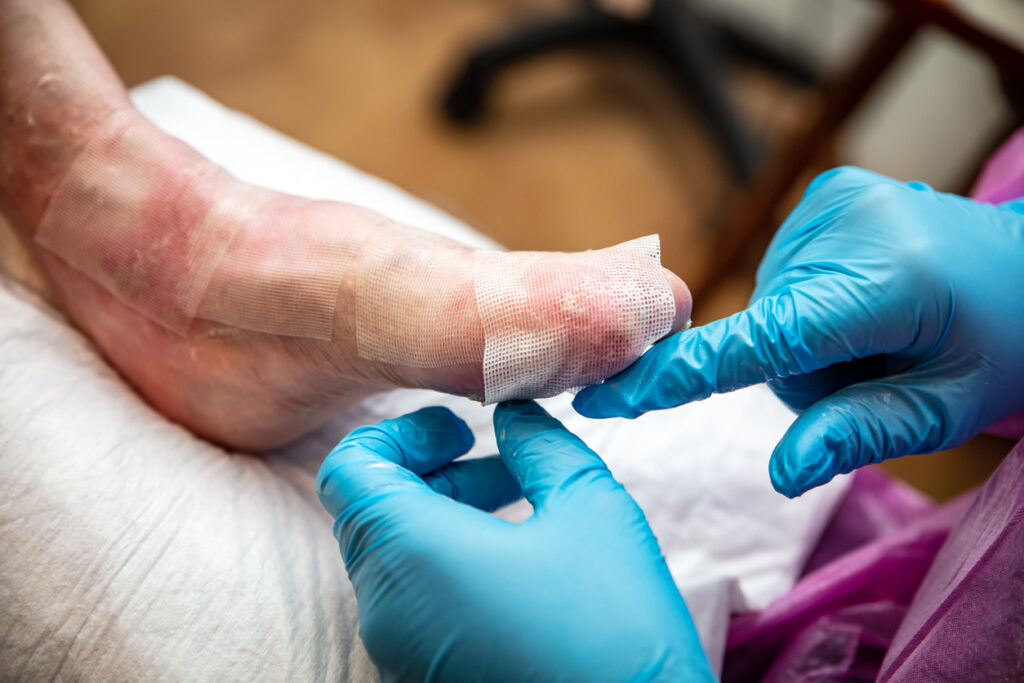
The Ann & Robert H. Lurie Children’s Hospital of Chicago is now activated as the first Qualified Treatment Center (QTC) for the use of Zevaskyn (prademagene zamikeracel) gene-modified cellular sheets to treat wounds associated with recessive dystrophic epidermolysis bullosa (RDEB).
Lurie Children’s has completed QTC start-up activities enabling it to begin patient identification for scheduling of Zevaskyn treatment. Treatments are expected to begin in the third quarter of 2025.
Abeona recently received approval from the U.S. Food and Drug Administration (FDA) for Zevaskyn as the first and only autologous cell-based gene therapy for the treatment of wounds in adult and pediatric patients with RDEB.
“Lurie Children’s is proud to be the first qualified treatment site in the U.S. to offer this groundbreaking treatment for RDEB patients,” says Amy Paller, MD, Head of the Epidermolysis Bullosa Research and Care Program at Lurie Children’s, and Chair of the Department of Dermatology at Northwestern University Feinberg School of Medicine in Chicago, Il, in a news release. “Grafting gene-corrected cellular sheets onto chronically open wounds of patients with RDEB promises the potential to provide long-term healing of wounds, reduction in pain and reduced risk of infection.”
Expanding Access to Zevaskyn
Abeona is committed to enabling access to Zevaskyn for eligible patients in the U.S. and has deployed services to provide information and resources to make informed decisions about treatment with Zevaskyn for RDEB wounds.
Abeona’s comprehensive patient support program, Abeona Assist™, offers personalized support, including helping patients understand their insurance benefits and financial assistance options, and providing travel and logistical assistance. For more information on how to access ZEVASKYN and learn about patient support services offered through Abeona Assist, visit www.abeonaassist.com, call 1-855-ABEONA-1 (1-855-223-6621) or email MyNavigator@AbeonaAssist.com.