Tampere University has licensed a drug molecule intended for treating Epidermolysis Bullosa (EB) to Theravia, a pharmaceutical company headquartered in Paris, France.
Tampere University and Theravia will develop, register and commercialize the drug. Theravia will be responsible for further drug development and conducting clinical trials in EB patients.
“Pre-clinical studies have shown that our drug molecule is more effective than any previously tested molecule for this rare skin disorder,” says Professor Tero Järvinen from Tampere University in Tampere, Finland, in a news release. “Pre-clinical studies have shown that our drug molecule is more effective than any previously tested molecule for this rare skin disorder.”
Based on the results of experimental disease models, both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have granted Orphan Drug (ODD) and Rare Pediatric Disease designations (RPDD) to the drug molecule.
The molecule was developed in the Laboratory of Regenerative Medicine, headed by Dr. Järvinen. The molecule is a recombinant fusion protein that was identified by screening billions of potential drug candidates. It comprises two mutually supportive functional units. The DecoDerma project, led by Dr. Järvinen and funded by Business Finland from 2021 to 2023, kick started the efforts to commercialize this molecule.
“I am proud that the molecule we discovered has been licensed. This is the result of 20 years of systematic research efforts. We hope the product will eventually help treat EB, which can be fatal in infancy. Without Theravia, we could not have progressed any further in the drug development using only our academic resources. Theravia possesses both the necessary skills and financial resources to carry out the clinical development,” Dr. Järvinen says.
“Theravia is especially enthusiastic about initiating this first scientific collaboration with Tampere University for the benefit of patients with Epidermolysis Bullosa,” says Franck Hamalian, CEO of Theravia. “Professor Järvinen and his team’s discovery holds great promise for EB patients worldwide. At Theravia, we are committed to addressing rare and neglected diseases such as EB, described by Debra America as ‘the worst disease you’ve never heard of.’”
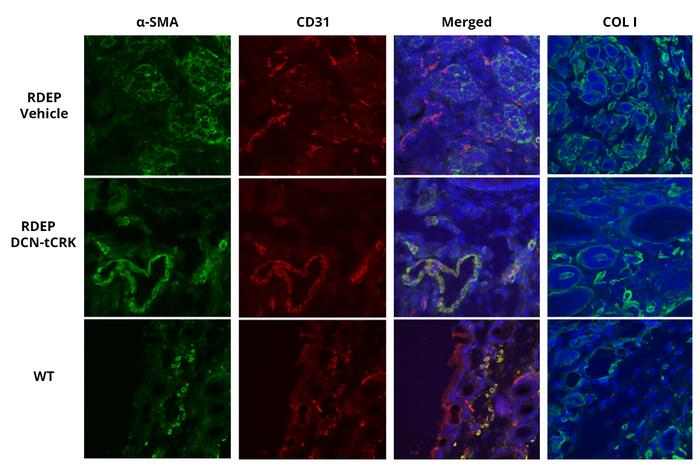
IMAGE CAPTION:
The drug inhibits progression of epidermolysis bullosa in skin. Recessive dystrophic pidermolysis bullosa (RDEB) leads to excessive deposition of fibrosis (COL I) in the skin in response to skin fragility. Fibrosis is produced by cells called myofibroblasts (α-SMA), which arise from normal fibroblasts. Both myofibroblasts (α-SMA) and fibrosis (COL I) formation can be inhibited by the drug (DCN-tCRK) developed by Tampere University Research team.
IMAGE CREDIT:
The original image published in Pemmari et al. Mol Ther 2020