U.S. FDA Grants Breakthrough Device Designation to Castle Biosciences’ DecisionDx-Melanoma Test
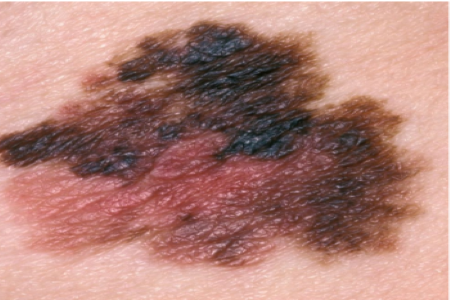
The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to Castle’s DecisionDx-Melanoma.

The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to Castle’s DecisionDx-Melanoma.