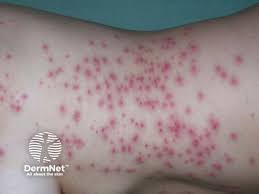
Lutris Pharma’s LUT014 gel showed significant efficacy in treating acneiform rash associated with the use of epidermal growth factor receptor (EGFR) inhibitor therapy, according to Phase 2 data presented at the European Society for Medical Oncology (ESMO) Gastrointestinal Cancers Congress 2025 in Barcelona, Spain.
LUT014 is a novel B-Raf inhibitor that is optimized for paradoxical mitogen‑activated protein kinase (MAPK) activation.
“EGFR inhibitors…are critical components of cancer therapy, but their effectiveness is often compromised by skin toxicities that can lead to dose modifications or treatment discontinuation,” says Benjamin W. Corn, MD, Chief Medical Officer of Lutris Pharma, in a news release. “LUT014 is designed to mitigate these toxicities by restoring downstream signaling in skin cells disrupted by EGFR inhibition.”
The trial enrolled 118 colorectal cancer patients from 23 clinical sites, all of whom had developed grade 2 or non-infected grade 3 acneiform rash while receiving cetuximab (Erbitux) or panitumumab (Vectibix). Participants were randomized in a 1:1:1 ratio to receive either LUT014 gel 0.03%, LUT014 gel 0.1%, or a placebo gel. The gel was applied daily for 28 days.
Primary Endpoint
The primary endpoint was the proportion of patients who achieved treatment success, measured by an improvement of at least one grade in Common Terminology Criteria for Adverse Events (CTCAE) scoring or an improvement of at least five points in the Functional Assessment of Cancer Therapy (FACT)-EGFRI-18 HRQoL skin-specific assessment. The study employed both an intention-to-treat (ITT) analysis and a Per-Protocol (PP) analysis (i.e., patients who dropped out or did not discontinue their EGFR inhibitor for reasons unrelated to the rash, such as disease progression were excluded from the analysis). Sample size calculation was based on an expected treatment success of 20% for the placebo group and 50% for one of the treatment groups.
The high dose of LUT014 demonstrated statistically significant rates of success improving acneiform rash in both the cetuximab and panitumumab ITT groups, compared to placebo. Additionally, patients randomized to LUT014 gel had lower rates of interruption of cetuximab or panitumumab therapy due to acneiform rash. In the open-label extension, even the low dose of LUT014 showed meaningful efficacy, with up to 69% of patients in the per-protocol population achieving improvement. LUT014 was also well tolerated, with fewer and mostly mild adverse events compared to placebo.
“We believe these results underscore LUT014’s potential to be a key therapeutic, offering significant benefits to patients by enabling them to remain on optimal anti-EGFR treatment without interruption,” Dr. Corn says.
PHOTO CREDIT: DermNet