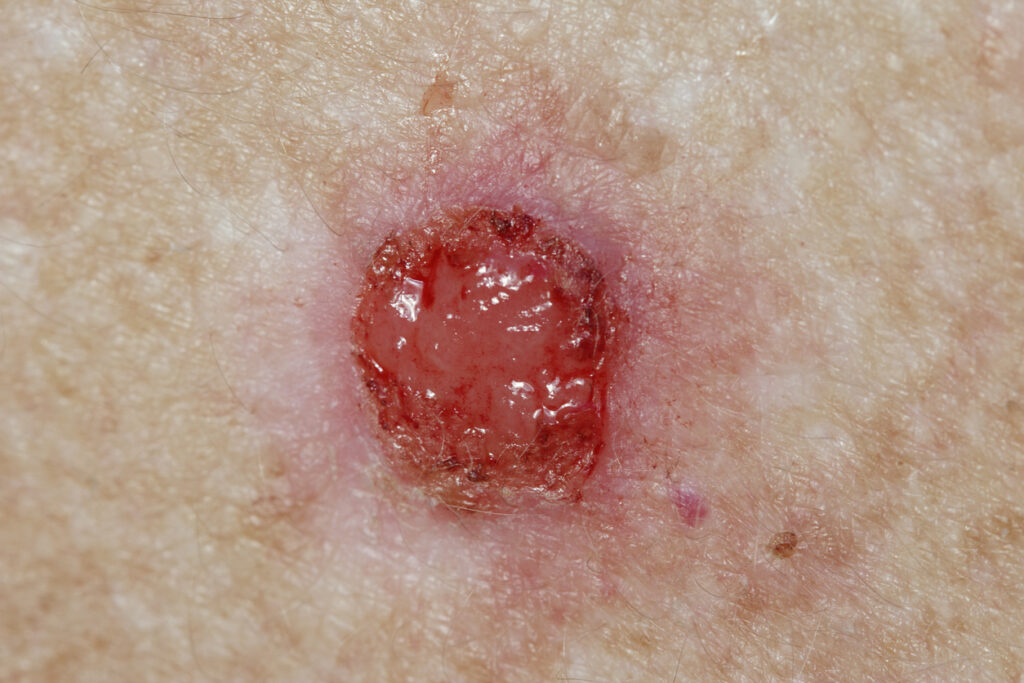
The first patient has been treated in Stamford Pharmaceuticals Inc.’s trial of SP-002, an adenoviral-based immunotherapy, and vismodegib (Erivedge, Roche) in locally advanced basal cell carcinoma (laBCC).
Vismodegib is a hedgehog Pathway inhibitor (HHPI) that is approved for the treatment of adult patients presenting with locally advanced and metastatic basal cell carcinoma.
SP-002 has been engineered to produce Interferon-g. Clinical studies with SP-002 alone have already been completed in cutaneous lymphomas, melanomas, and BCC. These studies demonstrated that SP-002 was safe, well tolerated and showed favorable clinical activity.
“We are excited to progress SP-002 in combination with HHPI into this subset of locally advanced BCC patients. This study was motivated by encouraging results observed in clinical studies evaluating SP-002 as a monotherapy or in combination with HHPI in other BCC settings. We believe there is an opportunity to further improve patient benefit in locally advanced BCC patients based on the current standard of care,” says Clement Leong, PhD, CEO of Stamford Pharmaceuticals, in a news release.
SP-002-004 is a multi-center randomized, phase 2 study that will enroll patients with locally advanced BCC patients. Patients will be randomized to receive oral vismodegib in combination with intratumoral injection of SP-002 or vismodegib alone. The primary objective is to determine overall response rate (ORR) for a target tumor following one or three cycles of SP-002 given as an add-on to HHPI in patients with laBCC. While the secondary objective is to determine the clinical benefit for a target tumor following one or three cycles of SP-002 given as an add-on to HHPI in patients with laBCC.