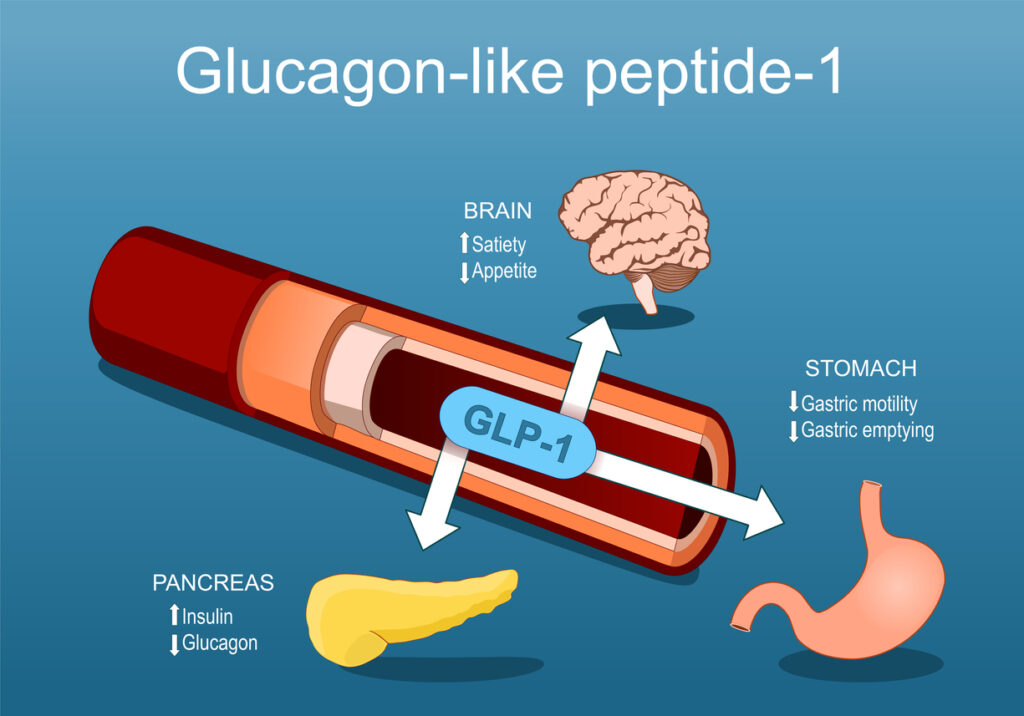
Women taking glucagon-like peptide-1 (GLP-1) receptor agonists are not at increased risk of developing acne, according to a new study in the International Journal of Women’s Dermatology.
For the study, researchers conducted a web-based analysis of six GLP-1 receptor agonists (three with a once-weekly dosing schedule and three with a once-daily dosing schedule) on PubMed. The meta-analysis included 45 research articles.
The use of dulaglutide, exenatide extended release, and semaglutide (Wegovy) showed no conclusive side effects from acne. In addition, no acne side effects were seen with the short-acting GLP-1 receptor agonists, including liraglutide, lixisenatide, and semaglutide (Rybelsus).
Three articles showed that GLP-1 agonists triggered adverse effects from the acne search criteria, such as a nodule or cyst, but they did not fulfill the criteria for a diagnosis consistent with acne vulgaris. Exenatide extended release was used in all three articles.
“It is unlikely that GLP-1 agonists themselves are directly responsible for the acne that some patients may develop during treatment,” the study authors note. “Rather, it is more probable that the weight loss yielded by treatment with these drugs may induce intrinsic physiologic and hormonal changes that induce or exacerbate acne vulgaris in such patients.”
The study did have its share of limitations. Specifically, there was a shortage of research regarding the relationship between GLP-1 agonists and acne.
“Future studies should analyze the dermatologic side effects of these medications as consumers share concerns regarding their impact on the skin,” the study authors conclude.