Helio Miot, MD, PhD, with Bob Kronemyer

HELIO MIOT, MD, PhD
Professor of Dermatology,
Unesp Botucatu,
Sao Paulo state, Brazil
The first head-to-head comparison study of cysteamine cream to hydroquinone for the treatment of recalcitrant melasma has found the relatively new topical agent to be safe, well-tolerated and effective, despite its inferior performance to hydroquinone.
The study, which was published last year in the International Journal of Dermatology, compared topical 5% cysteamine to 4% hydroquinone in treating facial melasma in 40 women.1
“The incidence of melasma is increasing among adult women all around the world,” said principal investigator Helio Miot, MD, PhD, a Professor of Dermatology at Unesp Botucatu, Sao Paulo state, Brazil, who noted that researchers have hypothesized that contraceptive pills, air pollution, and visible light exposure may be causing this increase.
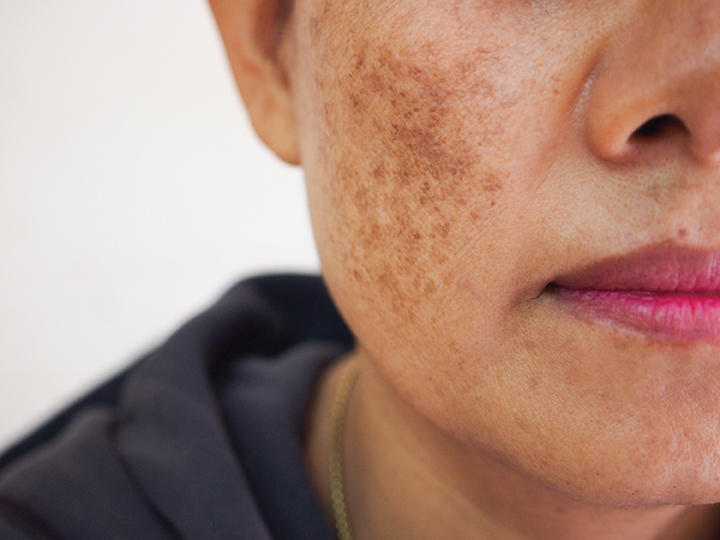
Melasma also has a high rate of recurrence, despite effective therapies like hydroquinone or triple combination cream (hydroquinone 5%, hydrocortisone 1%, and tretinoin 0.1%). “The majority of the effective depigmenting agents are tyrosinase inhibitors, of which hydroquinone is the most studied drug,” Dr. Miot said. “But new treatments are welcome, especially because melasma is relapsing.”
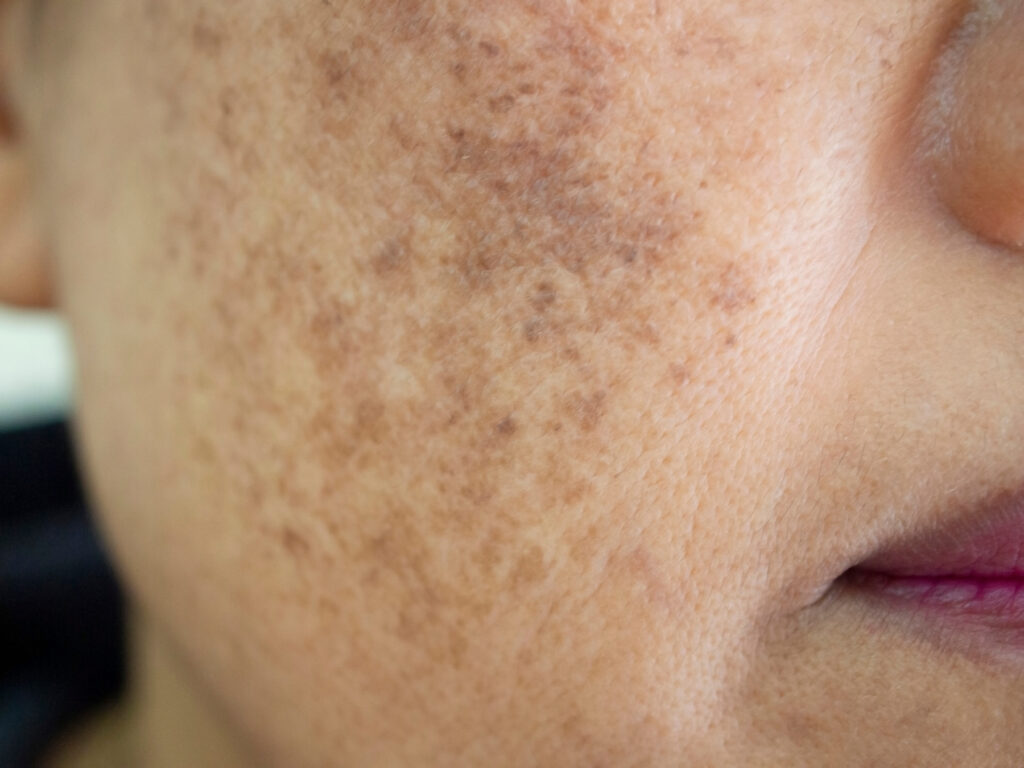
Melasma is a common chronic hypermelanosis that affects photoexposed areas, especially in women, and may have a negative effect on the patient’s quality of life.
However, because of the risk of exogenous ochronosis and “confetti” depigmentation as a result of the hydroquinone-induced melanocyte toxicity, “other potent depigmenting agents are frequently researched for the treatment of melasma,” Dr. Miot said. “Cysteamine is a potent bleaching agent with a different mode of action than other tyrosinase inhibitors.”
Cysteamine is an aminothiol compound naturally produced in the human body during the coenzyme A metabolism cycle and is a degradation product of the amino acid L-cysteine. “The agent’s mechanisms of action are not fully understood, though,” Dr. Miot said. “It is postulated that the skin lightening effect by cysteamine is due to its inherent antioxidant properties.”
The antioxidant also inhibits the melanin synthesis by decreasing the formation of dopachrome (the immediate precursor of eumelanin) and increasing intracellular glutathione, which shifts the eumelanin to pheomelanin synthesis, as well as to chelating copper ions required in melanogenesis.
“Despite having been discovered decades ago, the strong odor of cysteamine prohibited its use,” Dr. Miot said. “Fortunately, a recent change in the formulation has reduced the sulfur odor and skin irritability, allowing its use as a cream.”
Trial details
The quasi-randomized, multicenter, evaluator-blinded clinical trial from Brazil was conducted between October 2019 and February 2020, involving 40 women representative of a Brazilian sample of melasma patients, all of whom had facial melasma with skin phototype II to V, and aged 30 to 55.
Patients were divided into 2 groups of nightly drug application on hyperpigmented areas for 120 days: 5% cysteamine gel-cream (n = 20) and 4% hydroquinone (n = 20).
Both groups of women were also required to use tinted sunscreen with a sun protection factor (SPF) of 50 and a persistent pigment darkening (PPD) of 9.
Patients in the cysteamine group were instructed to maintain the cream on their skin for 15 minutes on the first night, followed by facial washing. Application time progressively increased up to 2 hours, if there was no skin irritation during the previous nights, again followed by facial washing.
The hydroquinone group was instructed to keep the cream on the face overnight.
Subjects were assessed at baseline and after 60 days and 120 days of treatment via the Melasma Area and Severity Index (mMASI) and the Melasma Quality of Life Scale (MELASQoL). Also assessed was the difference in colorimetric luminosity between melasma and the adjacent unaffected skin.
The Global Aesthetic Improvement Scale (GAIS) assessed the difference in the appearance of the skin through standardized photographs.
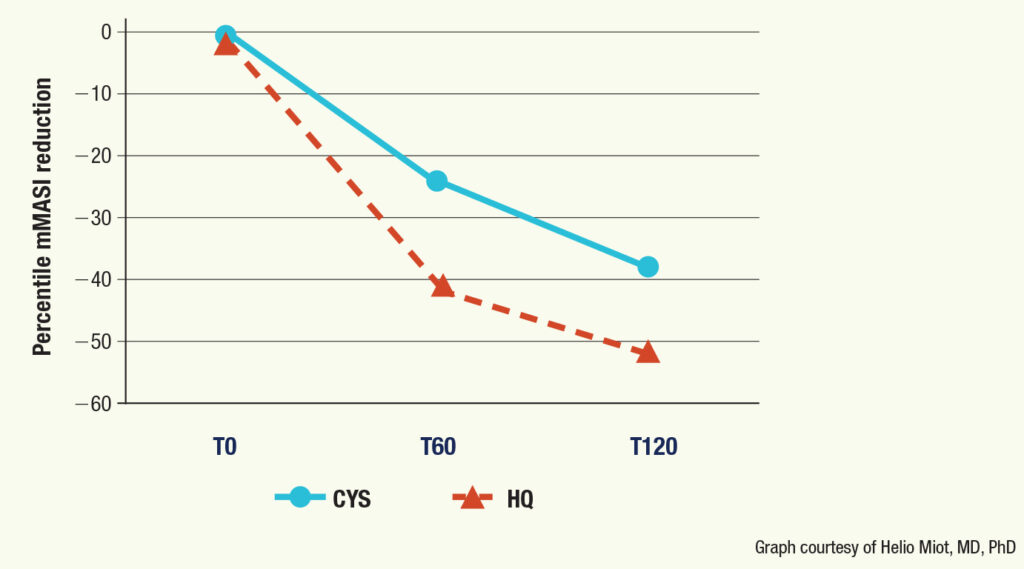
“Both groups exhibited a reduction in mMASI after 120 days of treatment,” Dr. Miot said. However, the improvement was greater in the group that received hydroquinone treatment: 41% vs 24% at 60 days, respectively, and 53% vs 38% at 120 days, respectively.
“The results of other trials reveal a mean 38% to 58% decrease in mMASI with topical cysteamine after 120 days, which is consistent with our outcomes,” Dr. Miot said.
The photographic evaluation concluded that there was up to 74% improvement in both groups, without a statistically significant difference between them (P = 0.087).
But MELASQoL favored hydroquinone: 20% vs 19% at 60 days, and 41% vs 27% at 120 days, respectively.
Colorimetric assessment revealed progressive depigmenting in both groups, again without a statistically significant difference between them (P > 0.160).
“The sulfur odor from the cysteamine was judged by the participants to be mild and completely subsided after facial washing,” Dr. Miot said. “But one patient reported headache because of the odor.”
There were no severe adverse effects related either to treatment or deviances in protocol as a result of skin intolerance. Erythema and burning sensation were the 2 most reported local adverse events in both groups. However, their frequency of up to 20% did not differ between the 2 groups.
Tolerability of erythema, desquamation, and burning also did not differ between the 2 groups (P > 0.17).
“Our trial confirms the efficacy and acceptability of a novel topical cysteamine formulation for the treatment of facial melasma, with the advantages of being safe, well-tolerated, and an option to patients that are intolerant or allergic to hydroquinone,” said Dr. Miot, who is not surprised by any of the study’s results. “Cysteamine has a different mode of action than hydroquinone 4% that has been proven to be effective.”
Limitations and possibilities
Three potential limitations of the study are that it was open, was performed in the summer, and had a relatively short follow-up period.
Dr. Miot said there are no reasons why hydroquinone or cysteamine should be used first. Using them in combination also has yet to be defined. “There is still no clinical trial evaluating the sequential or alternate use of these 2 drugs in melasma treatment,” he said.
The disadvantage of cysteamine, however, is the need to wash the applied area after 2 hours of treatment because there is a risk of itching and burning after long maintenance, according to Dr. Miot. “Most of our participants tolerated more than 1 hour of treatment, therefore the effect of overnight application should be assessed further,” he said.
Case reports indicate that cysteamine cream is effective for treating refractory post-inflammatory hyperpigmentation resistant to triple combination cream.
The next steps are to evaluate the long-term efficacy and tolerability of cysteamine, or perhaps to test the benefit of its association with other bleaching strategies, according to Dr. Miot.
Because melasma has a complex pathogenesis that surpasses melanocyte hypertrophy, a combination of strategies could lead to more effective outcomes. “For example, cysteamine might be positioned as a maintenance treatment after prescribing other potent depigmenting agents, microneedling, laser, or oral tranexamic acid,” Dr. Miot said.
REFERENCES
1. Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;August 31. doi:org/10.1111/ijd.15146
DISCLOSURES
Dr. Miot reports no relevant financial interests.