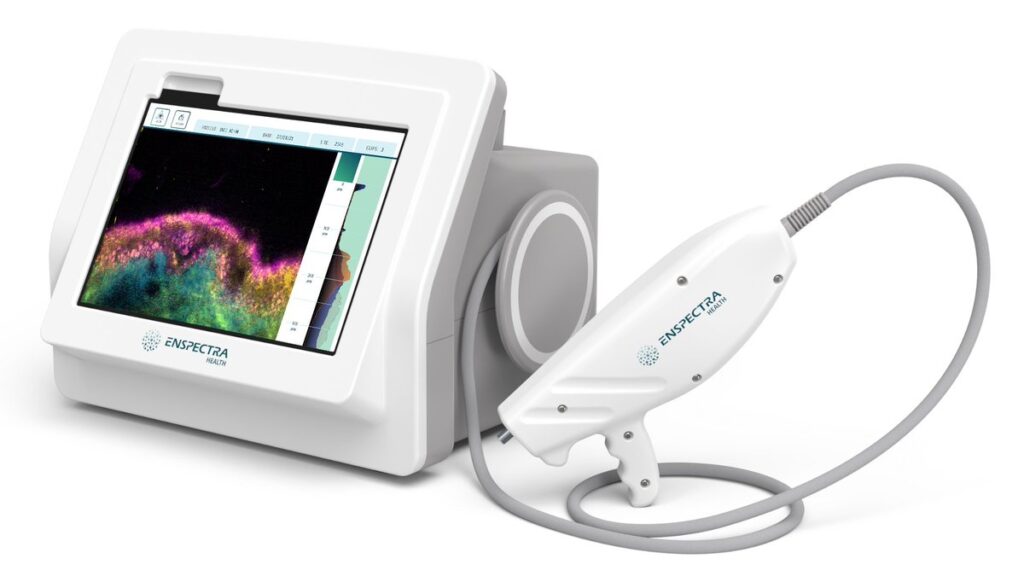
Enspectra Health’s U.S. Food and Drug Administration (FDA) cleared non-invasive cross-modal imaging technology (VIO System) provides real-time visualization of skin cellular structure and composition, according to research in Scientific Reports.
Portable and handheld, cross-model imaging digitizes histopathology directly from a patient’s skin to provide actionable health insights for dermatologists – without biopsy.
The publication outlines how cross-modal technology overcomes the limitations of prior imaging modalities such as monochromatic images and rigid positioning of patients. The cross-modal imaging technology delivers high-resolution, multi-color (Tetrachrome) results, making it practical for the in-office dermatology practice setting. The performance of the technology was evaluated with healthy human participants ranging in age from 9–81 years and in skin type diversity from Fitzpatrick I-V. Ethnic representation was a strength of the study; 43% of participants identified as Hispanic/Latino, Asian, Black/African American, or as multiracial.
Cross-modal images showed histological details analogous to those obtained from traditional biopsied tissue. In addition, study authors observed dermal elastosis in sun-damaged skin, elevated melanin in pigmented skin, basaloid nests in basal cell carcinoma, and elongated rete ridges in seborrheic keratosis, supporting cross-modal’s potential to deliver histological insights noninvasively.
“Our aim is to make the invisible digital by unveiling cellular and molecular information beneath the skin’s surface,” says Gabriel Sanchez, PhD, CEO and co-founder of Enspectra Health, in a news release. “We believe cross-modal imaging is a patient-centric platform that will usher in a new era of digital dermatology.”