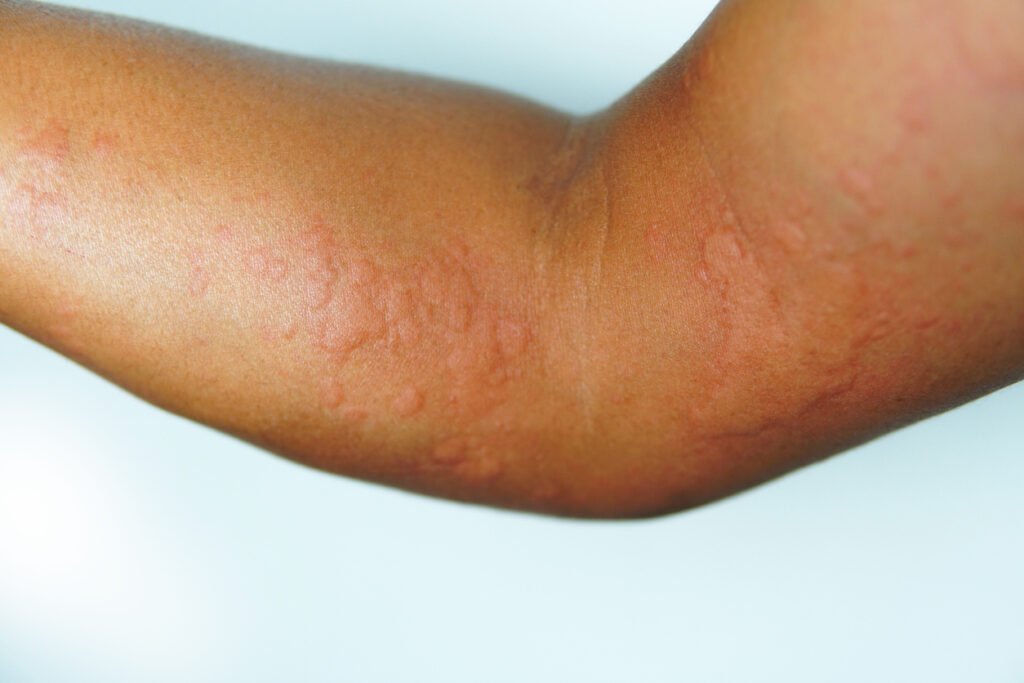
Remibrutinib (Rhapsido, Novartis) provides fast and sustained symptom relief for patients with chronic spontaneous urticaria (CSU) who remained symptomatic despite treatment with second-generation H1 antihistamines, according to a pooled analysis of REMIX-1/-2 studies presented at the 2025 American College of Allergy, Asthma & Immunology (ACAAI) Annual Scientific Meeting in Orlando, FL.
The U.S. Food and Drug Administration (FDA) approved remibrutinib for CSU in September 2025. It is the first FDA-approved Bruton’s tyrosine kinase inhibitor (BTKi) for CSU. IN REMIX-1/-2 studies, patients were randomized 2:1 to remibrutinib 25mg twice daily or placebo over a 24-week double-blind period, followed by a 28-week open-label remibrutinib treatment period. Placebo patients transitioned to remibrutinib at Week 24.
REMIX Study Investigator Michael J. Palumbo, MD, an allergist in Pittsburgh, PA, discussed the results of the pooled analysis and their implication for CSU patients with The Dermatology Digest.
TDD: What are the main takeaways from this pooled analysis?
Michael J. Palumbo, MD: “Total symptom scores in addition to urticaria index and itch severity were reduced substantially as early as Week 1, and many patients expressed improvement at Day 3 of treatment. This impact was evident in the quality of life, as measured by the Dermatology Life Quality Index (DLQI), at the first measurable time point (Week 4). A strong correlation was seen between Weekly Urticaria Activity Score (UAS7) and DLQI continued through Week 24.”
TDD: What type of improvements can CSU patients expect with this new treatment?
Dr. Palumbo: “Patients can expect to see rapid and sustained improvement in itch and hives, which continues to improve throughout 52 weeks. Up to 50% improvement was seen at Week 2, and a substantial number of patients continued to experience roughly 75% less severe hives compared to the start of the study at Week 52.”
TDD: How does CSU affect quality of life?
Dr. Palumbo: “CSU can have a profound impact on quality of life, interfering with everyday activities as patients are concerned with being seen in public while experiencing hives and disfiguring angioedema. Sleep is disrupted due to chronic itching of the skin. Subsequently, due to these disruptions in lifestyle, patients often see an increase in anxiety and depression when dealing with chronic hives. This is going to affect work and social life in addition to everyday activities.”
TDD: How is remibrutinib different from other CSU treatments?
Dr. Palumbo: “Remibrutinib is the first oral treatment of chronic spontaneous hives when antihistamines are not enough. It is not an antihistamine and not a steroid. The other currently approved treatments for CSU are injections. Remibrutinib works by acting directly on the mast cells to inhibit histamine release.”
TDD: Are CSU patients excited to have a new treatment option?
Dr. Palumbo: “My office was a site for the REMIX 1 and REMIX 2 clinical trials. It was rewarding to see patients, many of whom have been dealing with hives for years, finally see some relief of symptoms and in many cases within days of initiating treatment. These trials were interesting as it was very obvious from the onset which patients were in the active treatment group. Many patients entered the trial because they have a fear of needles and have been waiting for a safe oral option. It is great now to have more options for our patients which we can provide when having a shared decision discussion about next steps when high-dose antihistamines alone are not getting hives under control.”