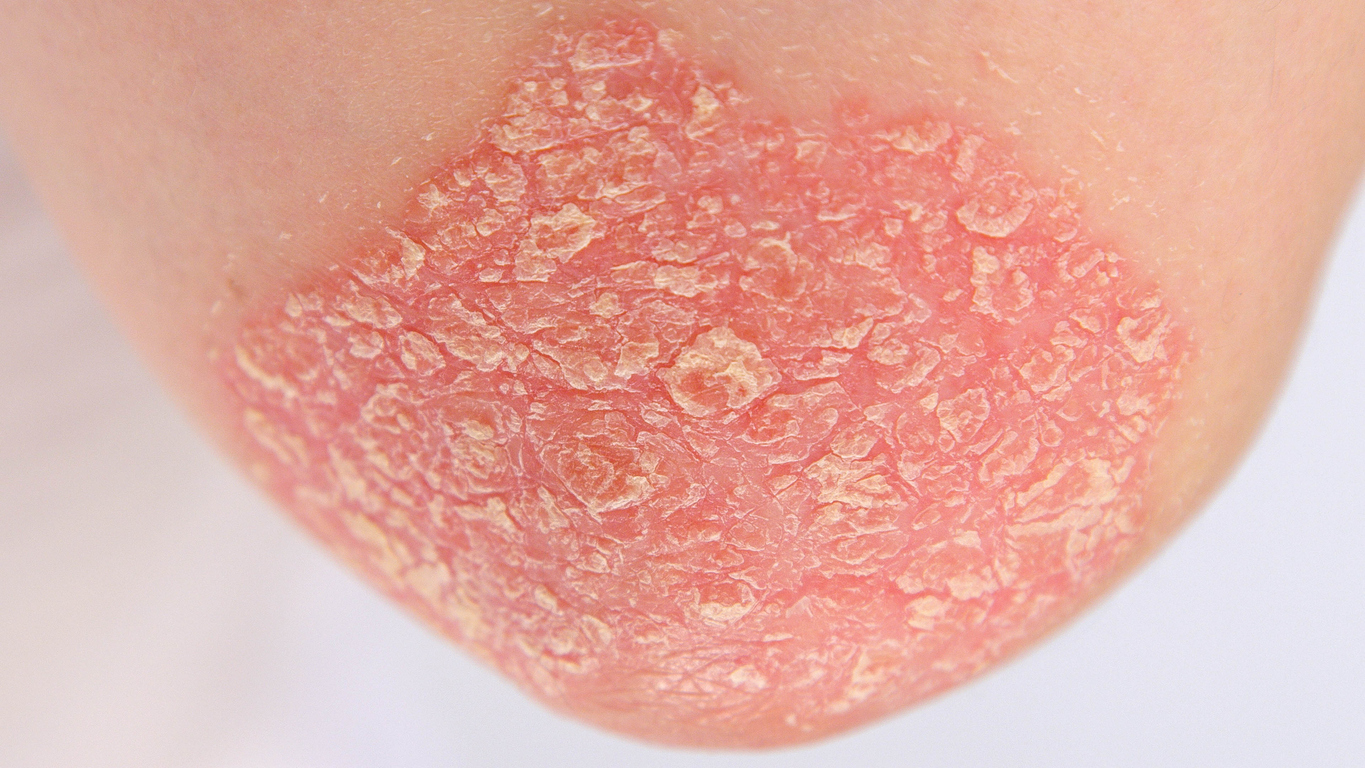
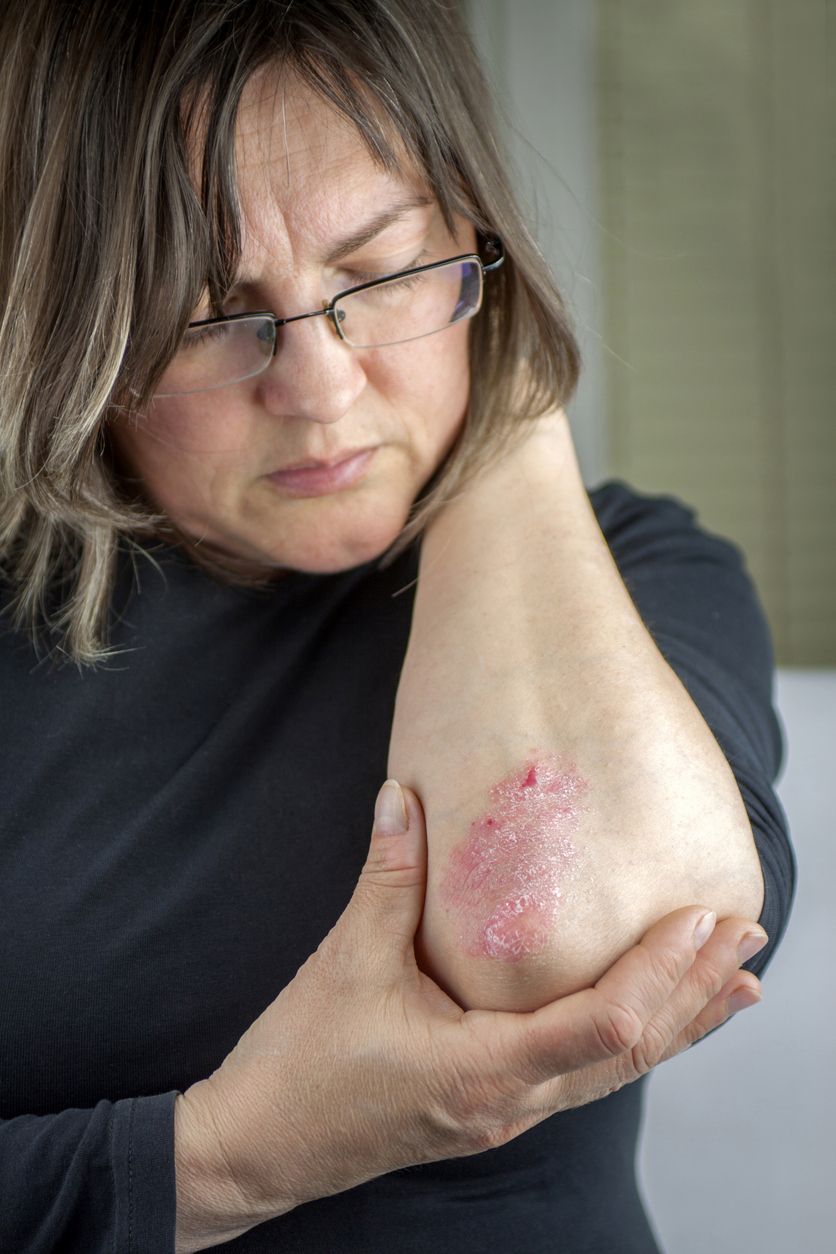
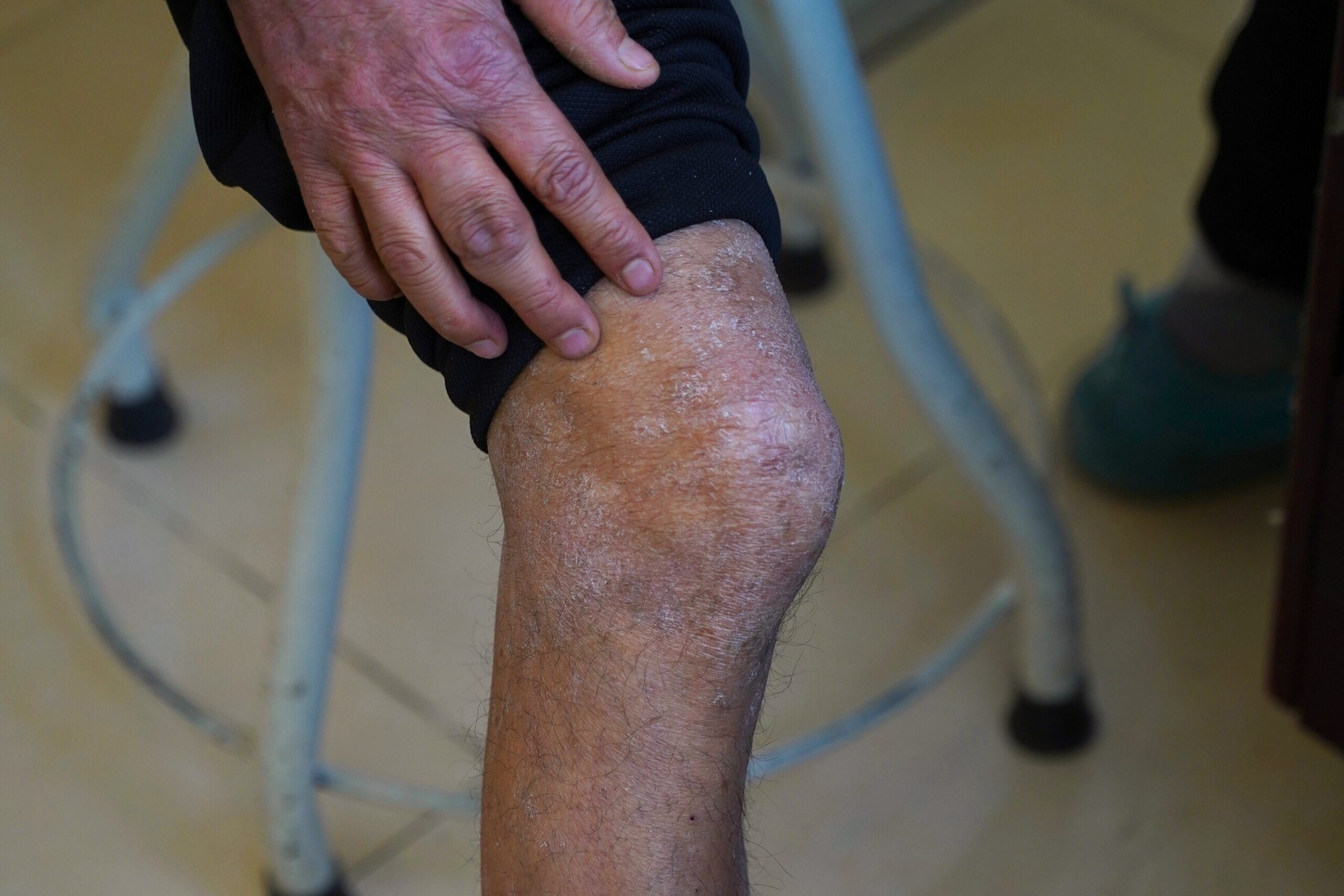
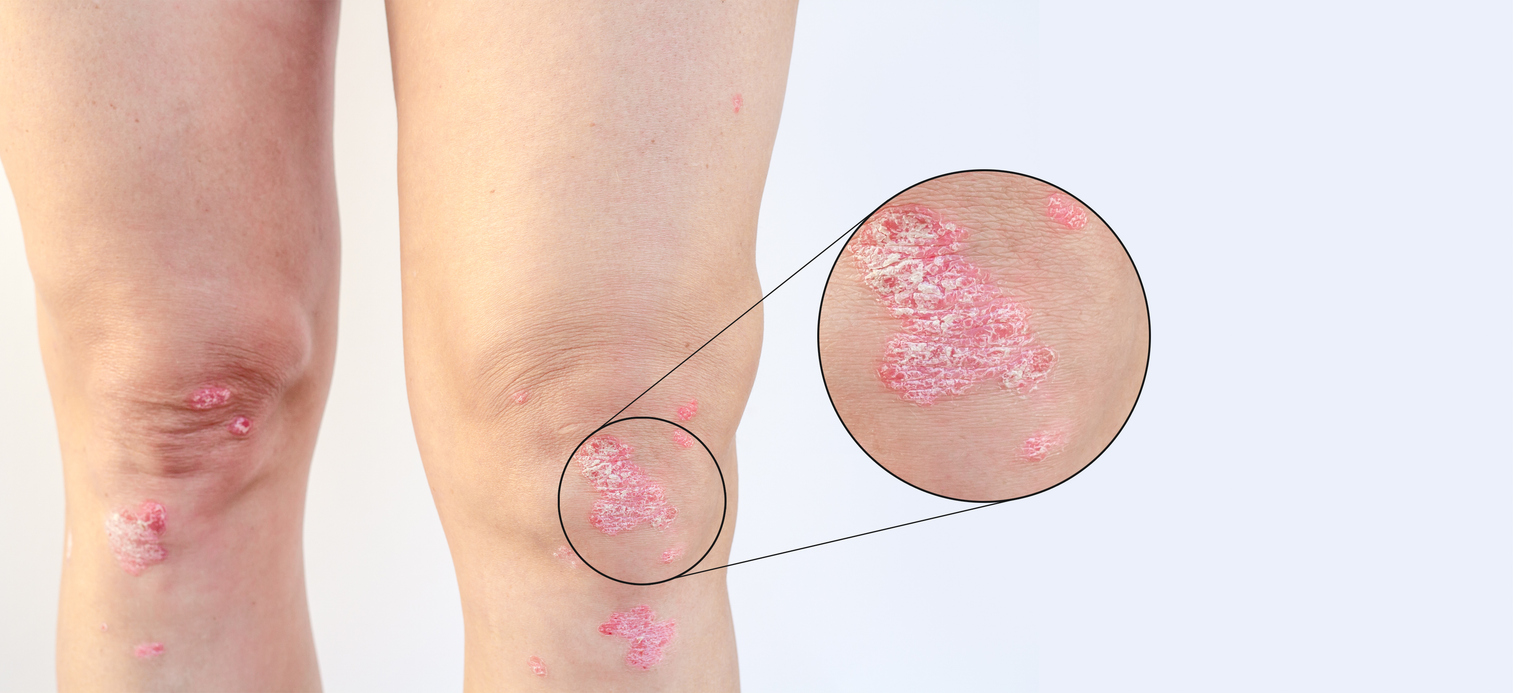
Psoriasis



Done Deal: LEO Pharma Closes Deal for BI’s Spesolimab (Spevigo)
October 2, 2025

U.S. FDA Approves Guselkumab for Pediatric Psoriasis and Active PsA
September 29, 2025

BMS Offers Deep Discounts on Deucravacitinib (Sotyktu)
September 28, 2025