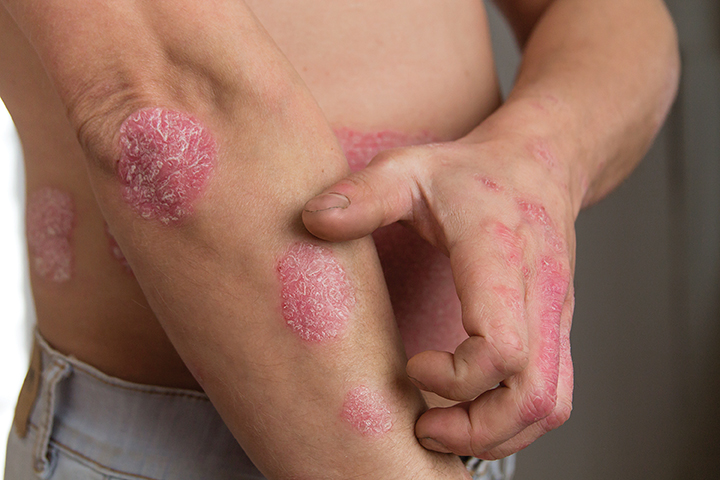
Protagonist Therapeutics, Inc.’s oral icotrokinra (JNJ-2113, formerly PN-235),performed well in two Phase 3 ICONIC studies.
Icotrokinra is the first-in-class targeted oral peptide that selectively blocks the interleukin (IL)-23 receptor, in individuals 12 years of age and older with moderate to severe plaque psoriasis. Protagonist Therapeutics and Johnson & Johnson have a license and collaboration agreement to develop and commercialize icotrokinra.
In the ICONIC-LEAD study, once-daily icotrokinra showed significant skin clearance versus placebo in adults and adolescents with moderate to severe plaque psoriasis. At week 16, nearly two-thirds of patients treated with icotrokinra achieved Investigator’s Global Assessment score (IGA) scores of 0/1 (clear or almost clear skin), and 49.6% achieved Psoriasis Area and Severity Index (PASI)=90[, compared to 8.3% and 4.4% on placebo, respectively. Further increases in response rates continued to be observed at week 24, with 74.1% of patients treated with icotrokinra achieving IGA scores of 0/1, and 64.9% achieving PASI-90.
Safety data was found to be consistent with the Phase 2 FRONTIER 1 and 2 studies. A similar proportion of patients experienced adverse events (AEs) between icotrokinra and placebo, with 49.3% and 49.1% of participants experiencing a treatment emergent adverse event (TEAE) at week 16.
In addition, positive topline results from the Phase 3 ICONIC-TOTAL study showed once-daily icotrokinra met the primary endpoint of IGA of 0/1 at week 16 compared to placebo.
“These positive Phase 3 results confirm the compelling efficacy and safety trends that were observed with the previous Phase 2b FRONTIER-1 and -2 studies, highlighting icotrokinra’s potential as a best-in-class oral agent providing an ideal combination of significant skin clearance with demonstrated tolerability in a once-daily pill for treating plaque psoriasis,” says Dinesh V. Patel, PhD, President and CEO of Protagonist, in a news release. “These results also continue to validate Protagonist’s innovative peptide technology platform and its effectiveness in creating highly differentiated new chemical entities to address unmet needs in various disease areas.”
Comprehensive results from both ICONIC-LEAD and ICONIC-TOTAL are being prepared for presentation at upcoming medical congresses and will be shared with health authorities in planned submissions.
Additional upcoming icotrokinra dermatology clinical studies and data anticipated in the first half of 2025 include:
- Topline results for the Phase 3 ICONIC-ADVANCE 1 and ICONIC-ADVANCE 2 superiority studies, evaluating the safety and efficacy of icotrokinra compared with both placebo and deucravacitinib in moderate to severe plaque psoriasis.
- An ICONIC-PsA psoriatic arthritis program evaluating icotrokinra in a phase 3 study in psoriatic arthritis (PsA) will be initiated in the beginning of 2025.
“We’re very pleased with the ICONIC-LEAD and ICONIC-TOTAL Phase 3 results, and the decision of our partner to initiate a Phase 3 program for icotrokinra in psoriatic arthritis,” adds Dr. Patel. “Our enthusiasm for icotrokinra is high heading into 2025, with Phase 2b ulcerative colitis results, presentation of ICONIC Phase 3 results at medical conferences, topline results from psoriasis superiority studies against deucravacitinib and a potential psoriasis NDA submission.”