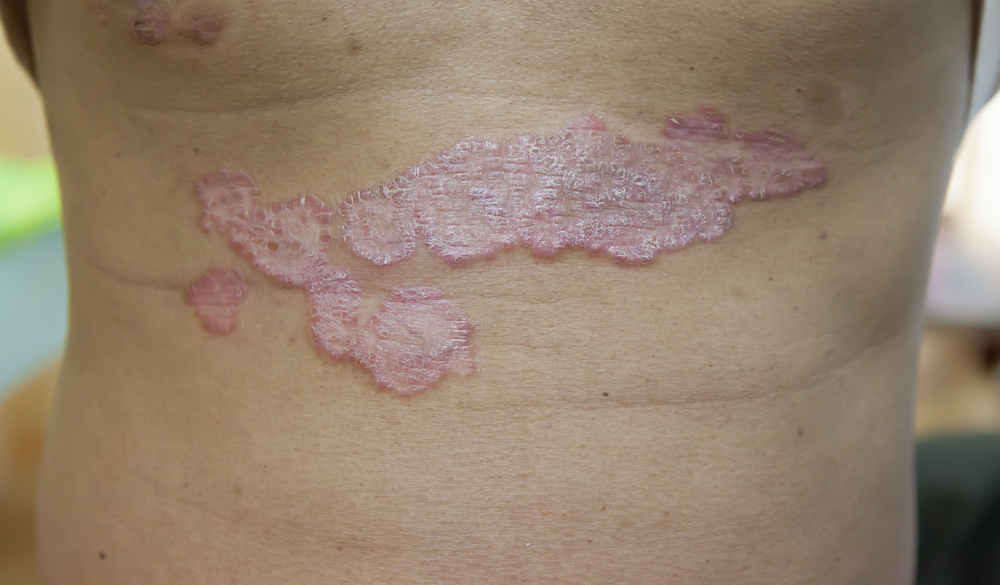
Alumis completed enrollment of more than 1,700 patients in its Phase 3 ONWARD clinical program for ESK-001, a next generation oral tyrosine kinase 2 (TYK2) inhibitor for the treatment of moderate-to-severe plaque psoriasis.
This will support a topline data readout early in the first quarter of 2026.
ESK-001, is a highly selective, next-generation oral TYK2 inhibitor that is designed to correct immune dysregulation across a spectrum of diseases driven by proinflammatory mediators, including IL-23, IL-17, and type 1 interferon (IFN).
Two Clinical Trials
The Phase 3 ONWARD clinical program consists of two parallel global Phase 3, multi-center, randomized, double-blind placebo-controlled 24-week clinical trials, ONWARD1 and ONWARD2, designed to evaluate the efficacy and safety of ESK-001 in adult patients with moderate-to-severe plaque psoriasis.
More than 1,700 patients were enrolled across the two trials and randomized 2:1:1 to receive ESK-001 40mg twice-daily, placebo or apremilast. The co-primary efficacy endpoints will be the proportion of patients with moderate-to-severe plaque psoriasis achieving a 75% improvement in the Psoriasis Area and Severity Index (PASI 75) and sPGA score 0/1 of ESK-001 compared to placebo at Week 16.
Patients completing Week 24 will have the opportunity to participate in a long-term extension trial, ONWARD3, that will evaluate durability and maintenance of response and long-term safety.
The Phase 3 clinical program is supported by positive data from the Phase 2 STRIDE clinical trial, and by the long-term open-label extension which is currently ongoing.
Once-Daily Formulation
In parallel with the Phase 3 clinical program, Alumis is developing a once-daily modified-release oral formulation of ESK-001 designed to replace the current immediate-release oral formulation that is dosed twice daily.
ESK-001 is also being evaluated in LUMUS, a Phase 2b clinical trial for the treatment of patients with systemic lupus erythematosus. In addition, Alumis continues to leverage its precision data analytics platform to explore ESK-001’s potential application in other immune-mediated conditions.