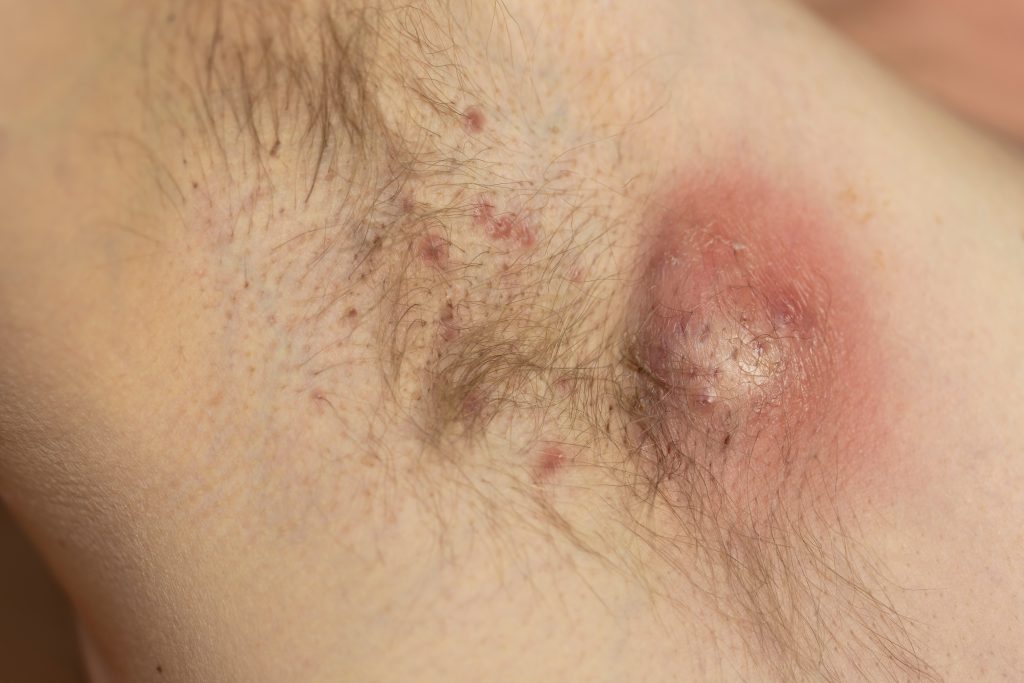
MoonLake Immunotherapeutics’s sonelokimab hit all endpoints in patients with moderate-to-severe hidradenitis suppurativa (HS), according to the Week 16 results of the Phase 3 VELA-1 and VELA-2 trials.
However, VELA-2 showed a higher-than-expected placebo response rate.
Sonelokimab is a nanobody designed to directly target sites of inflammation by inhibiting the interleukin (IL)-17A/A, IL-17A/F, and IL-17F/F dimers and to penetrate difficult-to-reach inflamed tissues.
The VELA program used the higher clinical response level of HS Clinical Response (HiSCR) 75 as the primary endpoint, which defines a response as an at least 75% reduction in abscess and inflammatory nodule count, with no increase from baseline in abscess or draining tunnel count.
Secondary Endpoints
Key secondary endpoints included the percentage of participants achieving HiSCR50 and the percentage of patients achieving a Dermatology Quality of Life Index (DLQI) total score reduction of >4 (minimal clinically important difference), among participants with a baseline DLQI >4, as well as other scores that reflect the evolving needs of HS patients, treating physicians, and regulators. These included the percentage of participants achieving at least a 55% reduction in the International HS Severity Scoring System (IHS4-55), the percentage of participants achieving at least a 3-point improvement from baseline in the worst pain Numerical Rating Scale (NRS) among participants with a baseline score of at least three points, and the change from baseline in the HS-specific Quality of Life score (HiSQOL).
A total of 838 patients were enrolled across both trials. The trials were identical in design, comparing a single 120mg dose of sonelokimab to placebo, with HiSCR75 reading out at Week 16. From Week 16, all patients receive the 120mg dose of sonelokimab through to 48 weeks, with a last assessment at Week 52, followed by an open-label extension for up to two years.
Protocol Design Consistent With MIRA
The Phase 3 program used a protocol design consistent with the Phase 2 MIRA trial, which identified the optimal dose of sonelokimab for HS. The VELA protocols and statistical analysis plans were prepared in accordance with regulatory agency advice and include two analysis strategies. The composite strategy for the VELA trials is the primary statistical analysis. The protocol specifies the treatment policy strategy as the alternative method of handling intercurrent events to test the robustness of the VELA data.
In the combined Phase 3 VELA program, all endpoints reached statistical significance, with p-values below 0.001, including lesion counts and patient-reported outcomes (PROs). Sonelokimab demonstrated the expected profile of response over time, with statistically significant HiSCR75 for both studies achieved as early as Week 4. A preliminary analysis suggests that responses continue to improve beyond Week 16 and that placebo patients crossing over at Week 16 achieve similar responses to those originally randomized to the sonelokimab 120mg arm, as of Week 20.
‘Higher Than Expected’ Response Rate
Using the treatment policy strategy as per protocol, both VELA-1 and VELA-2 demonstrated a statistically significant increase in the percentage of participants achieving HiSCR75 at Week 16, providing a clinically meaningful benefit. Response rates for sonelokimab 120mg were consistent between the two trials, with 34.8% and 35.9% of patients in VELA-1 and VELA-2 achieving HiSCR75 at Week 16, respectively. The placebo response rate in VELA-1 of 17.5% at Week 16 was within the historical Phase 3 range of 13% to 18%. The placebo response rate in VELA-2 of 25.6% at Week 16 was higher than expected.
Both VELA-1 and VELA-2 achieved statistical significance for all key secondary endpoints. This includes other lesion count-based endpoints (HiSCR50 and IHS4-55). It also includes relevant PROs in HS. Around 30% of patients experienced a marked reduction of pain, as measured by an at least three-point improvement in the worst pain NRS, in both VELA-1 and VELA-2. Sonelokimab showed a significant improvement of HiSQOL score at Week 16, which was consistent between VELA-1 and VELA-2. Almost 60% of patients achieved a meaningful (four points or more) improvement of DLQI, an approximately 20-percentage-point benefit over placebo.
The VELA program is conducted using a convenient subcutaneous dosing scheme with 1ml volume delivered every other week to Week 6 in the induction phase (four injections), and monthly from Week 8 for maintenance, with no up-titration. This profile is matched by improvements of HS lesions, including draining tunnels, as well as in all key PROs, such as quality-of-life and pain scores, that are meaningful for HS patients and their treating physicians.
Encouraged By Results
“We are encouraged by the results of VELA-1, which follow the expected performance of sonelokimab in all the important metrics for patients and treating physicians. The higher-than-expected placebo response rate in VELA-2 is disappointing, but we are encouraged by the consistent performance of sonelokimab arms across all endpoints in both studies,” says Prof. Kristian Reich, Founder and Chief Scientific Officer at MoonLake, in a news release.
We are pleased to see a favorable safety profile consistent with previous studies, with no new safety signals. We believe that this, together with the convenient dosing, the efficacy data in lesion-based metrics, and the patient-reported outcomes, shows the potential for a promising profile of sonelokimab in HS. Patients with HS are in desperate need of new treatment options, and we remain committed to our path forward in HS.”
Results Will Be Discussed With Regulators
These interim results will now be discussed with the relevant regulators, including the analytical strategies that consider the higher-than-expected placebo response rate in VELA-2 at Week 16, as well as the path to submission of a Biologics License Application.
The Company continues to progress with the development of its nanobody sonelokimab across a portfolio of indications, including:
- Q4 2025: Primary endpoint readout of the Phase 2 LEDA trial in palmoplantar pustulosis
- Q1 2026: Primary endpoint readout of the Phase 2 S-OLARIS trial in axial spondyloarthritis
- Q2 2026: 52-week data of the VELA-1 and VELA-2 trials in HS
- H1 2026: Primary endpoint readout of Phase 3 VELA-TEEN trial in adolescent HS
- H1 2026: Primary endpoint readout of Phase 3 IZAR program in psoriatic arthritis