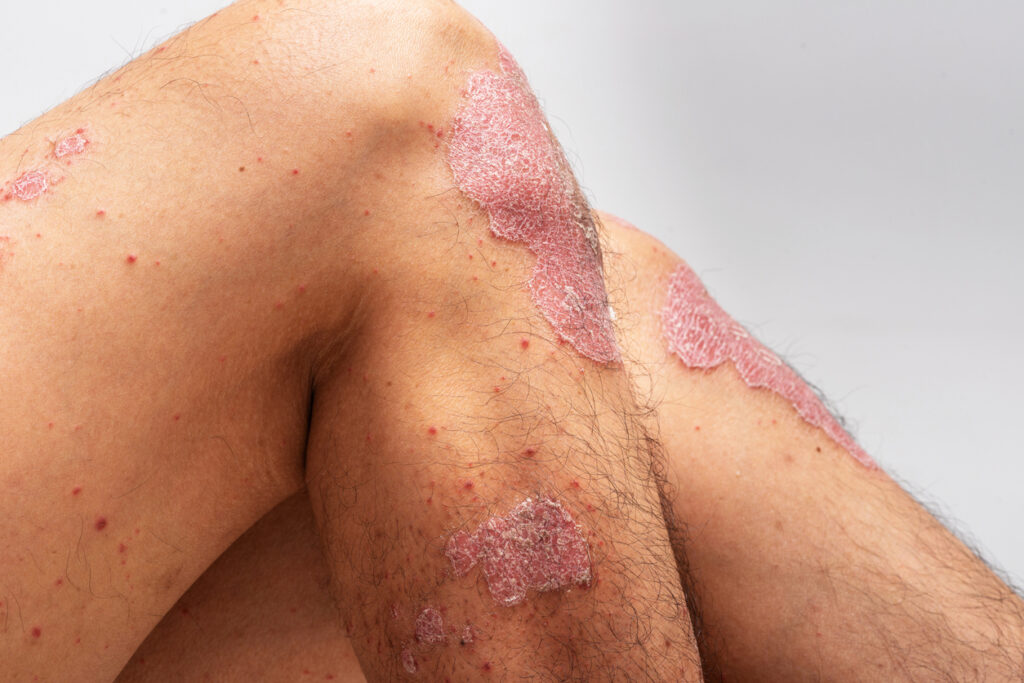
Icotrokinra, a first-in-class targeted oral peptide that selectively blocks interleukin (IL)-23, demonstrated significantly higher rates of skin clearance and a favorable safety profile in adults and adolescents with moderate-to-severe plaque psoriasis, according to the first Phase 3 study, which was presented at the 2025 American Academy of Dermatology’s annual meeting in Orlando, FL.
Protagonist Therapeutics and Johnson & Johnson have a license and collaboration agreement to develop and commercialize icotrokinra.
The pivotal, Phase 3, double-blind, placebo-controlled ICONIC-LEAD trial randomized 684 adults and adolescents (≥12-<18y) with moderate-to-severe plaque psoriasis i.e., a body surface area (BSA) of ≥10%; Psoriasis Area and Severity Index (PASI) of ≥12; overall Investigator’s Global Assessment (IGA) of ≥3) 2:1 to once-daily (QD) Icotrokinra, 200mg through Week24 or placebo through Week16 followed by transition to Icotrokinra, 200mg QD.
Co-primary endpoints were IGA0/1 (clear [0]/almost-clear [1] skin and ≥2-grade improvement from baseline) and PASI90 responses at Week 16. Fully 65% of Icotrokinra-treated patients achieved IGA0/1 compared to 8% of placebo-treated participants, and 50% of Icotrokinra-treated patients achieved PASI90 at Week16, compared to 4% of placebo-treated participants.
Icotrokinra completely cleared skin at significantly higher rates than placebo at Week16, the study showed. Moreover, Icotrokinra response rates continued to increase through Week 24, including IGA0/1 in 74%, PASI90 in 65%, IGA0 in 46%, and PASI100 in 40% of participants. Similar proportions of icotrokinra and placebo-treated participants had ≥1 adverse event (49% in each group; most commonly, nasopharyngitis and upper respiratory tract infection) and gastrointestinal-related events (6% per group) through Week 16. No safety signals emerged through Week 24.
ICYMI: Kenneth Gordon, MD, Professor and Chair of Dermatology at the Medical College of Wisconsin in Milwaukee, WI, reviews the “encouraging” efficacy data on icotrokinra at Maui Derm Caribbean 2025.
“It’s an oral drug that will rival biologics in efficacy,” says study author Jennifer Soung, MD, a Dermatologist and Director of Clinical Research at Southern California Dermatology in Los Angeles, CA. “Traditionally, orals have had less efficacy compared to biologics, but this is different. Now, I will be able to offer icotrokinra vs, injectable IL23 and not compromise efficacy.”
The hope is that it will be approved in adolescents at launch, she tells TDD. “This is amazing for kids who don’t want an injection.”
Icotrokinra has true game-changing potential, “It has excellent tolerability, no GI side effects OR nausea) and the safety is excellent,” she says, “Hopefully, minimal or no lab testing will be needed.”