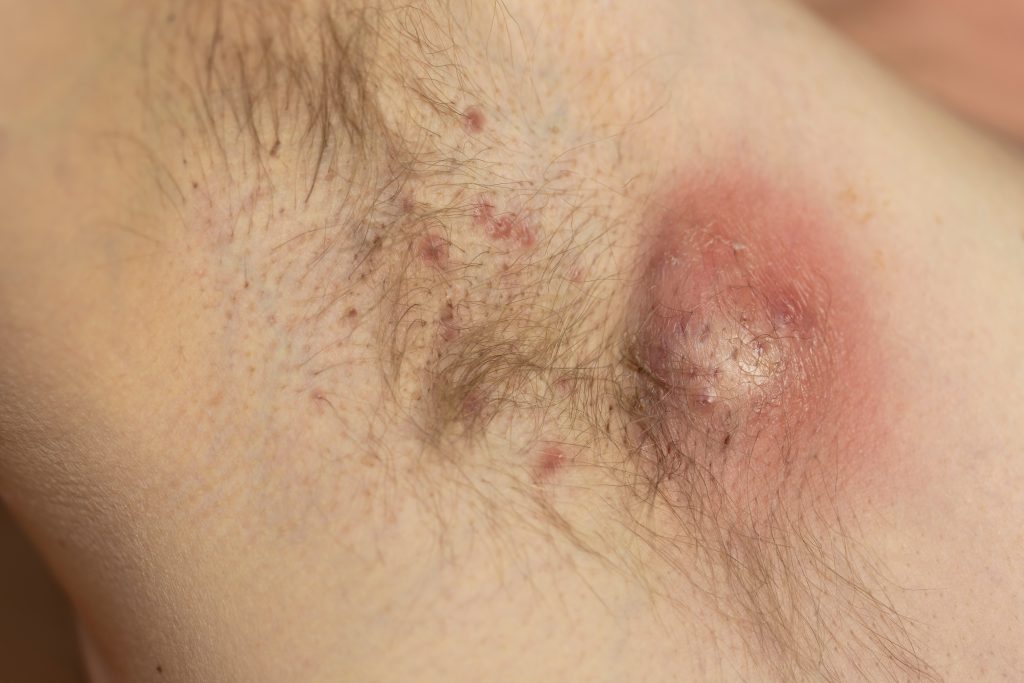
Bimekizumab-bkzx (Bimzelx, UCB) consistently showed sustained improvements in clinical and patient-reported outcomes in patients with moderate to severe hidradenitis suppurativa (HS), according to results from the Phase 3 BE HEARD I and BE HEARD II trials.
Bimekizumab-bkzx is an interleukin (IL)-17A and IL-17F inhibitor. The findings, which are published in the Lancet, support global regulatory submissions for bimekizumab-bkzx in HS.
“The Phase 3 studies with bimekizumab represent a significant milestone for the hidradenitis suppurativa community, and they include HiSCR75, a high threshold endpoint, as a key ranked secondary outcome. In these studies, bimekizumab consistently demonstrated sustained improvements in clinical- as well as patient-reported outcomes for people with moderate to severe disease. These findings provide strong support for targeting IL-17A and IL-17F as a new and promising therapeutic approach for the future.”
–Alexa B. Kimball, MD, MPH
“The Phase 3 studies with bimekizumab represent a significant milestone for the hidradenitis suppurativa community, and they include HiSCR75, a high threshold endpoint, as a key ranked secondary outcome,” says study author Alexa B. Kimball, MD, MPH, Beth Israel Deaconess Medical Center and Professor of Dermatology, Harvard Medical School, Boston, MA. “In these studies, bimekizumab consistently demonstrated sustained improvements in clinical- as well as patient-reported outcomes for people with moderate to severe disease. These findings provide strong support for targeting IL-17A and IL-17F as a new and promising therapeutic approach for the future.”
In April 2024, UCB announced that the U.S. Food and Drug Administration accepted for review the supplemental biologics license application for bimekizumab-bkzx for the treatment of adults with moderate-to-severe HS. The Company also announced that the European Commission granted marketing authorization for bimekizumab-bkzx for the treatment of active moderate-to-severe HS in adults with an inadequate response to conventional systemic HS therapy in April 2024. Other regulatory submissions for bimekizumab-bkzx in the treatment of moderate-to-severe hidradenitis suppurativa are underway around the world.
About BE HEARD I and BE HEARD II
BE HEARD I and BE HEARD II were randomized, double-blind, placebo-controlled, parallel-group, multicenter, Phase 3 trials designed to evaluate the efficacy and safety of bimekizumab in adults with moderate-to-severe HS. The two trials had a combined enrollment of 1,014 participants with a diagnosis of moderate-to-severe HS. The primary endpoint in both trials was HiSCR50 at Week 16. A key secondary endpoint was HiSCR75 at Week 16. HiSCR50 and HiSCR75 are defined as at least either a 50% or 75% reduction from baseline in the total abscess and inflammatory nodule count, with no increase from baseline in abscess and inflammatory nodule count or draining tunnel count.
Results from BE HEARD I and BE HEARD II showed that a significantly higher proportion of patients treated with bimekizumab versus placebo achieved a 50% or greater improvement in HS signs and symptoms at Week 16, as measured by HiSCR50, the primary endpoint in both trials. Bimekizumab treatment also resulted in clinically meaningful improvements in the ranked key secondary endpoint, HiSCR75 versus placebo at Week 16. Responses were maintained to Week 48. The safety profile of bimekizumab was consistent with safety data seen in previous trials with no new observed safety signals.