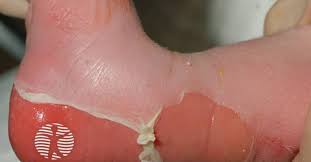
Rigosertib (Traws Pharma) is showing promise in patients with recessive dystrophic epidermolysis bullosa (RDEB)-associated cutaneous squamous cell carcinoma (RDEB SCC), according to a study in the British Journal of Dermatology.
People with RDEB are predisposed to develop highly aggressive SCC that is driven by overexpression of polo-like kinase 1 (PLK-1). There is a lack of effective prevention or treatment options for patients with RDEB-SCC. Rigosertib is a PLK1 inhibitor that is being developed as an oral and an intravenous formulation by Traws Pharma.
For the open-label, single arm Phase 2 studies, five RDEB SCC patients were offered either oral or intravenous administration of rigosertib. Patients were monitored with clinical photography, biopsy, PET-CT scans, and quality of life questionnaires over the 12 months’ duration of the trial. Pharmacokinetics of drug absorption was monitored in four patients.
RDEB SCC patients on intravenous or oral therapy showed anti-tumor efficacy with acceptable toxicity, and two patients had a complete response within six months of treatment. There was an overall response rate of 80%, with a complete response rate of 50%.
Quality of life was not negatively impacted by treatment, and drug absorption exceeded previous patient populations presumably due to the relatively high dosing in an underweight patient cohort, the researchers report.
A Potential Treatment
“These data indicate that rigosertib is a potential treatment for cutaneous SCC in RDEB patients, where there is a substantial unmet need and no approved therapies. The aggressive course of this disease is inadequately addressed by current treatment regimens, which produce limited response rates of mostly short duration,” says Victor Moyo, MD, the Chief Medical Officer Oncology at Traws Pharma, in a news release.
“We are excited to report the compelling efficacy and tolerability profile of rigosertib in this devastating, difficult-to-treat disease, and thank the patients, sponsors, and investigators for their commitment to this program,” says Iain Dukes, MA, DPhil, Interim CEO at Traws Pharma. “Rigosertib is available for further development and commercialization, and we are committed to finding an appropriate partner to advance this important medicine to approval.”
Traws Pharma stock surged after the Phase 2 results were released, according to media reports.
PHOTO CREDIT: DermNet