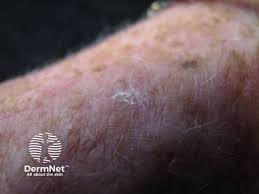
Biofrontera Inc. has completed enrollment in its Phase 3 clinical trial evaluating aminolevulinic acid hydrochloride (Ameluz) with photodynamic therapy (PDT) for the treatment of mild to moderate actinic keratoses (AKs) on the extremities, neck, and trunk.
With enrollment complete, Biofrontera anticipates finishing the treatment phase of the study by September 2025 and the follow-up phase by Q2 2026.
Multicenter, Randomized Trial
This trial is a multicenter, randomized, double-blind study comparing aminolevulinic acid hydrochloride with vehicle in the field-directed treatment of AK located on the extremities, neck, and trunk with PDT using a RhodoLED lamp (BF-RhodoLED XL or BF-RhodoLED). It is designed to assess the safety and efficacy of aminolevulinic acid hydrochloride PDT following the application of one to three tubes of product over a surface area (continuous or in patches) of approximately 80, 160, or 240cm2.
Patients receive one PDT treatment with either aminolevulinic acid hydrochloride or vehicle gel, and a second one at 12 weeks if at least one AK lesion remains. They are then followed up for approximately one year after the last PDT treatment. The study enrolled 172 patients who received either Ameluz® or vehicle gel in a ratio of 4:1.
“Ameluz PDT has already proven to be a valuable option for the treatment of AKs on the face and scalp,” says Nathalie Zeitouni, MD, a Mohs surgeon and principal investigator at Medical Dermatology Specialists and Professor of Dermatology at the University of Arizona COM Phoenix, in a news release. “We see many patients who have these lesions on other areas of the body, and the possibility of expanding the use of Ameluz to treat those areas is promising for both physicians and for our patients. I look forward to seeing the results of this study.”
Pending positive outcomes, the company plans to submit a supplemental New Drug Application (sNDA) to the U. S. Food and Drug Administration (FDA) in the second half of 2026.
PHOTO CREDIT: DermNet