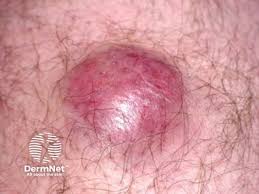
TuHURA Biosciences, Inc. is initiating a Phase 1b/2a trial of IFx-Hu2.0 in combination with pembrolizumab(Keytruda, Merck) when administered via Interventional Radiology (IR) in metastatic Merkel Cell carcinoma of unknown primary origin (MCCUP).
These patients have advanced or metastatic MCC with deep-seated tumors without associated cutaneous tumors and would not be eligible for the Company’s planned Phase 3 accelerated approval trial, which is targeted to begin enrollment later in Q2 2025.
IFx-Hu2.0 is designed to overcome primary resistance to checkpoint inhibitors (CPIs) and has demonstrated systemic anti-tumor specific immune responses (abscopal effect) when injected intratumorally into cutaneous, subcutaneous, or accessible nodal lesions in its prior Phase 1 and 1b trials in melanoma and advanced or metastatic MCC.
Multicenter, Open-Label Trial
The Phase 1b/2a trial of IFx-Hu2.0 is a multicenter, open-label trial designed to assess the safety and feasibility of IFx-Hu2.0 as adjunctive therapy to pembrolizumab in adult patients with non-cutaneous MCC. The trial is designed to enroll a total of nine non-cutaneous MCC patients with either hepatic, pulmonary, or retroperitoneal lesions, enrolling three patients per lesion type. Each patient will receive IFx-Hu2.0 (0.1mg) injected into a single visceral tumor once a week for three weeks. Within 48 hours of the first IFx-Hu2.0 injection, patients will receive pembrolizumab, followed by pembrolizumab every three weeks for six months.
The primary endpoint of the study is safety and feasibility of IFx-Hu2.0 adjuvant therapy evaluated 28 days following the last dose of IFx-Hu2.0, or Day 49 from the first IFx-Hu2.0 infusion. The study will also evaluate key secondary endpoints, including efficacy per RECIST 1.1 criteria at three months and six months. Data from the trial is anticipated by the end of Q4 2025 or early Q1 2026.
Phase 3 Trial
TuHURA is also preparing to initiate a single, randomized, placebo-controlled Phase 3 accelerated approval trial of IFx-2.0 administered as an adjunctive therapy to pembrolizumab vs. pembrolizumab plus placebo in first-line treatment for checkpoint inhibitor-naïve patients with advanced or metastatic MCC. The primary endpoint in the Phase 3 trial will be overall response rate (ORR) at approximately 24 weeks, with a secondary endpoint of progression free survival (PFS) per RECIST 1.1 criteria.
The Company has reached agreement with U.S. Food and Drug Administration (FDA) on requirements for lifting a partial clinical hold on this trial and believes it will meet the requirements in Q2 2025. TuHURA currently anticipates initiating the Phase 3 registrational trial in Q2 2025.
Based on data generated in TuHURA’s Phase 1b trial of IFx-Hu2.0 in CPI naïve patients with advanced or metastatic MCC who progressed receiving CPI therapy, FDA’s Oncology Center of Excellence (OCE), consistent with the FDA’s Project Front Runner Initiative, asked the Company to consider a first-line randomized placebo-controlled trial of pembrolizumab plus placebo or IFx-Hu2.0. The FDA also agreed that the trial could be conducted under their accelerated approval pathway with the use of Objective Response Rate (ORR) as the primary endpoint.
Key Secondary Endpoint
The FDA also asked the Company to consider incorporating into its accelerated approval trial a key secondary endpoint of Progression Free Survival (PFS), which, if successfully achieved without a detriment to overall survival at the time of analysis, may result in the Company not being required to conduct a post approval confirmatory trial, and this single trial could potentially fulfill requirement for regular approval. The Company and the FDA have entered into a Special Protocol Assessment Agreement for the planned Phase 3 trial.
“Like our planned Phase 3 accelerated approval trial, this Phase 1b/2a trial will also investigate the ability of IFx-Hu2.0 to increase the anti-tumor response rate when used alongside Keytruda in first-line treatment of CPI naïve, metastatic MCC. However, unlike the planned Phase 3 study, these are patients without skin lesions who present with metastatic deep-seated tumors in the liver, lungs or retropertitoneum (abdomen),” says James Bianco, MD, President and Chief Executive Officer of TuHURA Biosciences, in a news release. “Up to 30% of patients with MCC present without primary lesions in the skin, so this trial will not only provide safety, feasibility, and efficacy data, but may also expand the potential number of addressable patients who may benefit from IFx-Hu2.0.”
Extend Enrollment
If feasibility and safety is demonstrated for IFx-Hu2.0 and Keytruda® when radiologically administered to deep-seated tumors, the Company will extend enrollment to a variety of non-MCC cancers that are known not to respond or respond poorly to CPIs.
“Since the underlying biology of why tumors don’t respond to CPIs is for the most part the same, then the mechanism of how IFx-Hu2.0 overcomes that resistance to CPIs should be independent of the type of cancer treated,” Dr. Bianco says. “We have previously demonstrated that IFx-Hu2.0 can overcome CPI resistance in melanoma, squamous cell, and Merkel cell carcinoma, three unrelated types of skin cancers. If successful, this trial could expand the potential benefit of IFx-Hu2.0 to a wide variety of cancers.”
PHOTO CREDIT: DermNet