Veradermics has completed an oversubscribed $150 million Series C financing.
The financing was designed to support the registrational development and planned New Drug Application (NDA) submission for Veradermics’ lead investigational product, VDPHL01, the potential first and only extended-release oral minoxidil treatment designed specifically for hair regrowth in women and men.
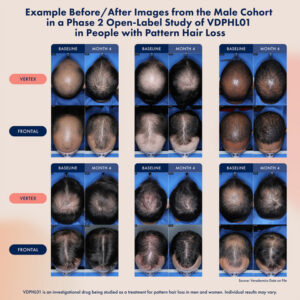
In related news, preliminary data from the male cohort from its ongoing Phase 2 trial of VDPHL01 showed visible and measurable regrowth as early as two months.
Financing Update
The financing was led by SR One, with participation from new investors Viking Global Investors, Marshall Wace, Invus, funds managed by abrdn Inc., Columbia Threadneedle Investments, Infinitium, LifeSci Venture Partners, and current investors including Longitude Capital, Suvretta Capital Management, Surveyor Capital (a Citadel company), and other undisclosed investors. In conjunction with the financing, Katarina Pance, PhD, an investor at SR One, has joined the Veradermics’ Board of Directors.
“We believe VDPHL01 represents the rare convergence of scientific innovation, favorable preliminary clinical data, and potential commercial opportunity. For the first time, we’re seeing an oral therapeutic candidate designed specifically for hair regrowth that has the potential to achieve consistent efficacy without compromising safety,” says Pance in a news release. “We believe that VDPHL01, if approved, can represent a front-line product for one of the largest aesthetics conditions worldwide. We are very proud to back Veradermics as they advance a program that could meaningfully impact both patient outcomes and the dermatology market at large.”
Proceeds from the financing are intended to advance and complete the ongoing Phase 3 trials and, if the clinical data are supportive, a subsequent NDA submission to the US Food and Drug Administration (FDA) for VDPHL01.
Filling a Void
Immediate-release oral minoxidil, a blood pressure medication that is not FDA-approved as a treatment for hair regrowth and is used off-label, is associated with an efficacy ceiling that limits hair-regrowth potential, a short plasma half-life that leads to rapid elimination of the majority of the drug in approximately two hours after dosing, and dose-limiting tolerability, including cardiac toxicities, associated with drug-concentration spikes immediately after administration.
Topical minoxidil 5% has also shown limited efficacy, and nearly 90% of patients discontinue use due in part to its messy application.
Veradermics utilized a proprietary extended-release technology to develop VDPHL01, an oral tablet designed to extend exposure of minoxidil to hair follicles over time. This release profile is intended to enable fast, consistent, and intense hair growth, while avoiding concentration spikes above minoxidil’s identified cardiac activity threshold.
“As a dermatologist, I’ve seen firsthand the emotional toll of pattern hair loss and watched as patients have had to settle for inconvenient, poorly tolerated, or off-label, non-clinically validated treatments,” says Reid Waldman, MD, Chief Executive Officer of Veradermics. “We built Veradermics to change that. With VDPHL01, we’ve engineered an extended-release oral formulation of minoxidil that we believe can maximize hair-growth potential while minimizing cardiac risks to safely regrow hair. We’re thrilled to see early indications of this playing out in the clinic, with preliminary Phase 2 data in males on VDPHL01 that indicate visible, measurable regrowth as early as two months.”
More About the Ongoing Phase 2 Trial
Veradermics is evaluating the safety and efficacy of VDPHL01 in an open-label, multi-dose Phase 2 trial of women and men with mild-to-moderate pattern hair loss who are not receiving any other active treatment, with primary endpoints of non-vellus target area hair count (and patient-reported outcomes on VDPHL01 effectiveness).
Dosing is ongoing in both female and male study participants. The male cohort was enrolled first, and preliminary results at two and four months, following a four-month treatment period, are currently available only for the male cohort.
Among 21 male participants in the Phase 2 trial who received VDPHL01 8.5 mg twice daily (BID) for two months, participants achieved an average increase in non-vellus target area hair count of 37.5 hairs/cm2 from baseline. At four months, the same participants achieved an average increase in non-vellus target area hair count of 47.3 hairs/cm2 from baseline. In addition, 55% of these males reported seeing much “improved” or “much improved” hair coverage in the same two-month period, increasing to 90.5% at four months. At the end of this four-month period, 95.0% expressed increased satisfaction in their hair coverage. Importantly, VDPHL01 has been generally well-tolerated to date and has not been associated with any serious adverse events, including any cardiac adverse events.
Phase 3 Trials Underway for Women and Men
Veradermics has initiated three multicenter, randomized Phase 3 clinical trials of VDPHL01 in males (NCT06724614, NCT06972264) and females (NCT07146022) with pattern hair loss. For more information about enrollment, please visit www.phlstudy.com or www.phlstudy.com/female.