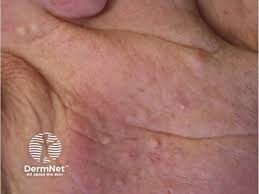
The U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for Azitra, Inc.’s topical ATR-04 to treat moderate to severe Epidermal Growth Factor Receptor inhibitor (EGFRi) associated dermal toxicity.
ATR-04 is a live biotherapeutic product candidate containing an isolated, naturally derived Staphylococcus epidermidis strain that was engineered to be safer by deleting an antibiotic resistance gene and engineering auxotrophy to control the growth of ATR-04. ATR-04 is in development for EGFRi-associated skin rash, which is caused by the suppression of skin immunity and subsequent inflammation and often elevated levels of IL-36γ and S. aureus. Approximately 150,000 patients are affected by EGFRi-induced skin toxicity in the United States.
Azitra plans to initiate a multicenter, randomized, controlled Phase 1/2 clinical trial of ATR-04 in patients with dermal toxicity due to EGFR inhibitors by the end of 2024.
“Many cancer patients receive EGFR inhibitors, which often have significant side effects, resulting in rashes that require off-label treatment with antibiotics, steroids or other medications, or discontinuation of EGFRi therapy,” says Francisco Salva, Azitra’s CEO, in a news release. “The skin toxicity creates a high burden for these cancer patients, with a profound impact on their quality of life. We look forward to potentially accelerating the development of ATR-04 to treat this condition.”
PHOTO CREDIT: DermNet