LEO Pharma recently acquired TMB-001 and other assets from Timber Pharmaceuticals following Timber’s Chapter 11 bankruptcy filing.
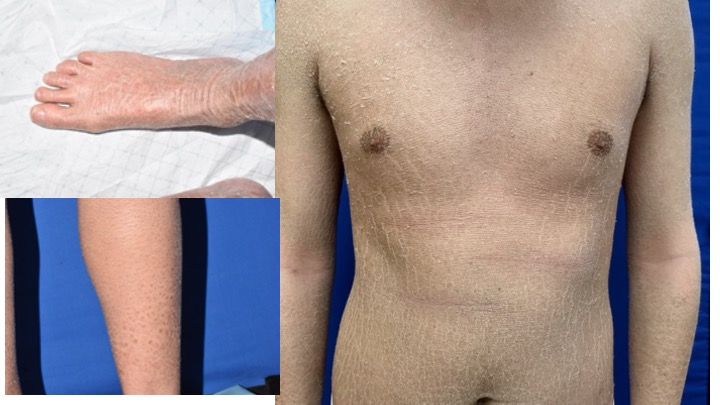
TMB-001 is an investigational topical reformulation of isotretinoin that is being studied in congenital ichthyosis (CI). The reformulation has received orphan, fast track, and breakthrough designation by the FDA. TMB-001 has shown positive results in Phase 2, with Phase 3 recruitment currently ongoing in the US.
Brian Hilberdink, LEO Pharma’s EVP of North America, chatted with TDD about the acquisition and how it fits in with LEO Pharma’s larger plans. Here’s what he had to say:
What are the next steps with TMB-001 for congenital ichthyosis (CI)?
Mr. Hilberdink: “We are finishing the recruitment for the Phase 3 trial and once completed, plan to submit the new drug application (NDA) for FDA approval.. The trial includes patients with lamellar ichthyosis (LI) and X-linked ichthyosis (XLI). If and when we receive FDA approval, we will consider this treatment as a potential part of lifestyle management for CI. This is an innovative topical isotretinoin that is being investigated for application on a large body surface. With our history and heritage in terms of dermatology and how to formulate topicals, we believe we are a good owner for this asset and will significantly help these patients who otherwise wouldn’t have any solutions.”
How do you see the outlook changing for ichthyosis patients in the coming years?
Mr. Hilberdink: “CI is a horribly debilitating disease. The hope is that after the completion of the successful Phase 3 trial and NDA approval by FDA, we can dramatically impact the quality of life for these individuals who experience physical and psychological pain. The ability to provide health care providers with an approved treatment for CI is something that we believe will be game-changing.”
Will we see a focus on orphan diseases from LEO Pharma?
Mr. Hilberdink: “LEO Pharma is a company that truly aspires to be a leading medical dermatology company that looks at unmet needs in the marketplace, and we see an unmet need in congenital ichthyosis (CI). The fact that we can potentially bring the first U.S. Food and Drug Administration (FDA)-approved treatment for CI is in the wheelhouse where LEO Pharma likes to play. Yes, as part of our mission, we are going to continue to look at other hard-to-treat and rare medical dermatology diseases. We are looking to move into chronic hand eczema which is another underserved disease state.”