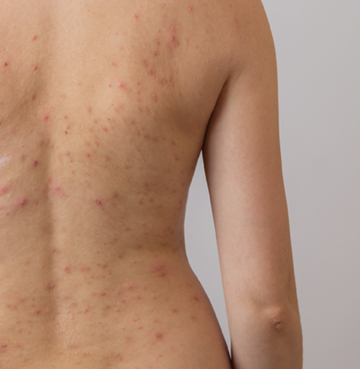
A novel topical BRAF inhibitor gel called LUT014 may significantly reduce the severity of acneiform rashes, common and painful side effects experienced by patients undergoing anti-epidermal growth factor (EGFR) therapies for colorectal cancer, according to a new study presented at the annual American Association for Cancer Research (AACR) meeting in Chicago, IL.
“The findings offer the first real solution in two decades for managing this rash, which frequently impacts patients receiving targeted therapies for colorectal cancer,” says study co-author Zev Wainberg, MD, professor of medicine at the David Geffen School of Medicine at UCLA, in a news release. “The ability to effectively treat it with a simple topical gel has the potential to greatly improve quality of life for patients and treatment outcomes.”
Reactivates MAPK Signaling Pathway
LUT014, being developed by Lutris Pharma, works by paradoxically reactivating MAPK, a key signaling pathway in the skin that anti-EGFR therapies shut down. By applying the BRAF inhibitor gel directly to the affected areas, the gel helps restore skin function, reduce inflammation, and improve symptoms without impacting the anti-cancer effects of the treatment.
The Phase 2 double-blind, placebo-controlled, and randomized study enrolled 118 patients across 23 medical centers. Patients in the study had colorectal cancer and developed moderate-to-severe rashes while taking cetuximab (Erbitux, Eli Lilly) or panitumumab (Vectibix, Amgen), two common anti-EGFR treatments.
The participants were randomly divided into three groups. One group used a low-dose gel, another a higher-dose gel, and the third used a placebo gel, which had no active drug. In each case, the gel was applied once a day for 28 days.
The main goal was to see if the rash improved, either by one level of severity or through better quality-of-life scores related to skin issues. The researchers found patients using LUT014 gel experienced marked improvements in rash severity and quality of life compared to those receiving a placebo, without interfering with their cancer treatment.
Higher Dose Improves Scores
Nearly 70% in the group using higher-dose gel saw their scores improve compared to about half (48%) of those using the low-dose gel and about one in three (33%) patients using gel with no active drug, the study showed.
“Until now, patients were simply told that the rash was an unavoidable side effect of these treatments, something they had to endure for the sake of fighting their cancer,” says Antoni Ribas, MD, PhD, professor of medicine at the David Geffen School of Medicine and director of the tumor immunology program at the UCLA Health Jonsson Comprehensive Cancer Center, and a study co-author. “But the data is overwhelmingly positive, and this approach not only improves patients’ quality of life but also makes the cancer treatment more manageable.”