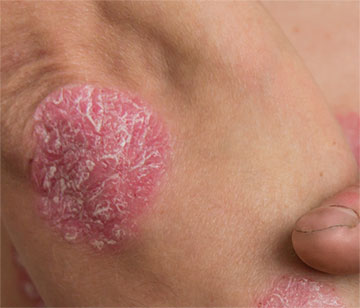
The National Psoriasis Foundation has expanded its Seal of Recognition to include U.S Food and Drug Administration (FDA)-approved prescription drugs to better represent the full toolbox of options available for people with psoriatic disease. The first such Seal of Recognition goes to roflumilast cream 0.3% and roflumilast topical foam 0.3% (Zoryve, Arcutis), both FDA approved for the treatment of plaque psoriasis.
Expand Beyond Over-the-Counter
“We are pleased to expand our directory beyond over-the-counter items to recognize FDA-approved treatments like this one. It is great for our community to have multiple formulations that are suitable for sensitive and hard-to-treat areas. This reflects real progress in addressing the daily needs of people with psoriasis,” says Leah M. Howard, JD, President and CEO of the NPF, in a news release.
The program was launched in February 2012 and has grown over time, with NPF now adding new products regularly. The decision to expand the directory to include FDA-approved products helps health care providers and people with the disease to see the full array of options for addressing psoriatic disease and its impactful symptoms.
A Tremendous Honor
“It is a tremendous honor that ZORYVE is the first FDA-approved product to receive the NPF Seal of Recognition. This is a testament to the dedication of our research, development, and technical operations teams to develop advanced targeted topical therapies,” adds Frank Watanabe, President and CEO of Arcutis.
“Individuals living with psoriasis want steroid-free treatments that are safe and effective for long-term use, as well as convenient and versatile. With cream and foam formulations, ZORYVE offers individuals with psoriasis and their physicians a choice of their preferred formulation of ZORYVE, each providing powerful, long-term relief of plaques and itch anywhere on the body—including thin-skinned and hair-bearing areas—with no limitation on duration of use. This recognition reinforces our commitment to developing innovative therapies that meet the real-world needs of people with chronic inflammatory skin diseases.”