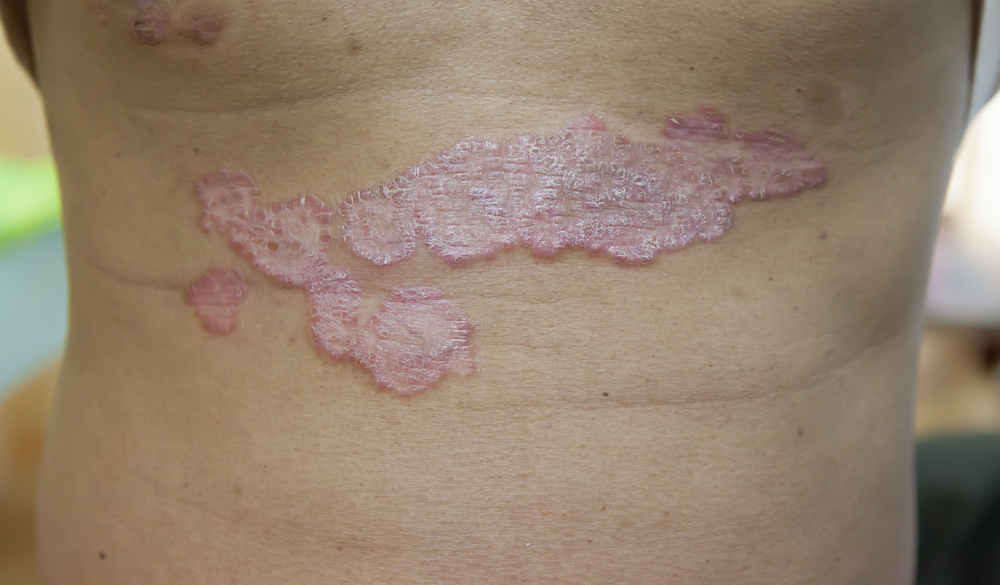
Risankizumab (Skyrizi, AbbVie) achieved significantly higher durable clinical responses in real-world practice compared to a BioCohort, including other biologics, for the treatment of moderate-to-severe psoriasis, according to a 148-week interim analysis of the ongoing VALUE trial presented at the World Psoriasis & Psoriatic Arthritis (IFPA) Conference in Stockholm, Sweden.
In the post-marketing study of more than 2,600 patients, those receiving risankizumab achieved complete/near complete skin clearance and maintained response in higher proportions than patients in the Biocohort. Specifically, 67.2% of people taking risankizumab achieved a Psoriasis Area and Severity Index (PASI)-90 compared with 45.3% in the BioCohort. Fully 55.2% of people taking risankizumab hit PASI 100, compared with 34.6% in the BioCohort. Patients receiving risankizumab also changed treatment significantly less often and achieved higher Treatment Satisfaction Questionnaire for Medication (TSQM) scores, the study showed. Other Effectiveness endpoints included Dermatology Life Quality Index (DLQI), and changes to treatment. The DLQI in the risankizumab group was significantly lower than the OtherBios group, and significantly fewer patients in the risankizumab group changed treatment compared to the OtherBios group, the study showed.
Risankizumab’s safety profile was similar or favorable when compared with OtherBios, the study showed.
ICYMI: Psoriasis Update: Can We Finally Say Cure?