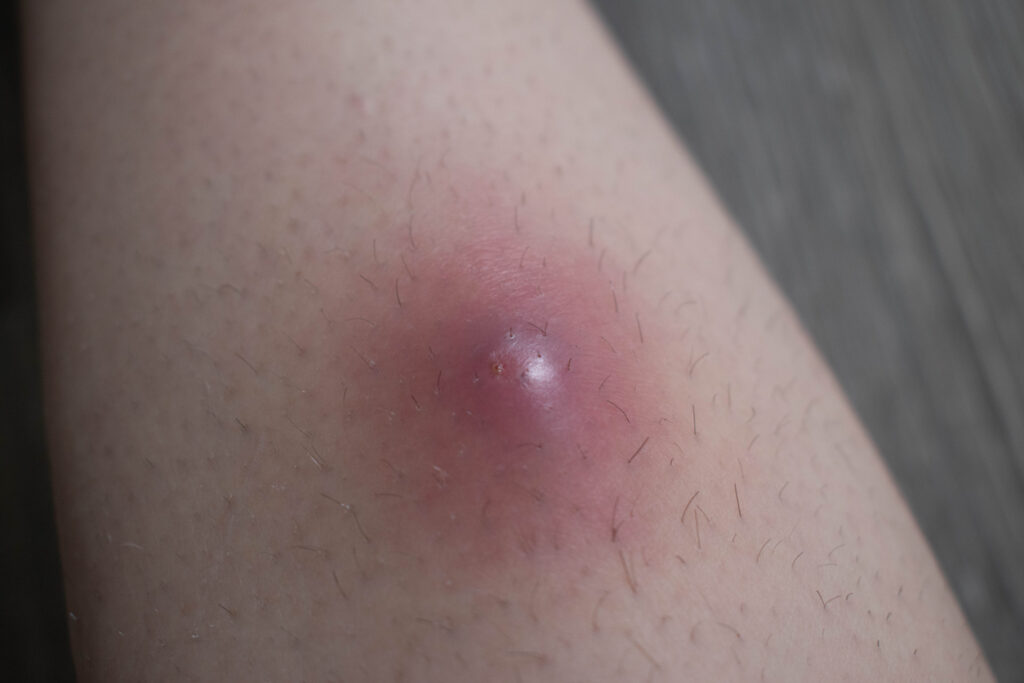
Bimekizumab (Bimzelx, UCB) shows sustained efficacy in moderate to severe hidradenitis suppurativa (HS), according to data from the BE HEARD trials presented at the 2025 European Hidradenitis Suppurativa Foundation conference in Vilnius, Lithuania.
Bimekizumab selectively inhibit both interleukin 17A (IL-17A) and interleukin 17F (IL-17F) .
Among HS patients who achieved response to bimekizumab at one year, over 85.0% maintained response across a range of endpoints to two years (percentage responders at Week 48 through Week 96: 90.0% (332/369) maintained Hidradenitis Suppurativa Clinical Response (HiSCR)50; 86.9% (265/305) maintained HiSCR75; 86.0% (234/272) maintained Dermatology Life Quality Index (DLQI) minimal clinically important difference (MCID) MCID).
Improvements in disease severity, as measured by I international Hidradenitis Suppurativa Severity Score System (IHS4) were demonstrated with the majority of patients shifting over two years from severe to mild and moderate disease (baseline to two-year data: mild: 0.0% to 53.1% (237/446); moderate:12.6% (70/556) to 26.5% (118/446); severe: 87.4% (486/556) to 20.4% (91/446), the studies showed.
The mean draining tunnel count also decreased over two years. Additionally, patients reported that gradual improvement to no/mild severity of symptoms translated to improved health-related quality of life over two years.
“For people living with HS, draining tunnels associated with moderate and severe disease can be incredibly distressing and painful – often derailing daily life,” says Professor Christos C. Zouboulis, President of the European Hidradenitis Suppurativa Foundation (EHSF) e.V., Director of the Departments of Dermatology, Venereology, Allergology and Immunology, Städtisches Klinikum Dessau, and Founding Professor of Dermatology and Venereology at the Brandenburg Medical School, Germany, in a news release. “These new, specific two-year data demonstrate bimekizumab’s ability to provide sustained disease control, meaning a shift towards mild disease characterized by the absence of draining tunnels, offering hope for long-term disease management and reduced burden for HS patients.”
The abstracts will be presented as two posters and three oral presentations at the 2025 Conference. Further results from BE HEARD EXT evaluating the efficacy and safety profile of bimekizumab will be presented later this year.