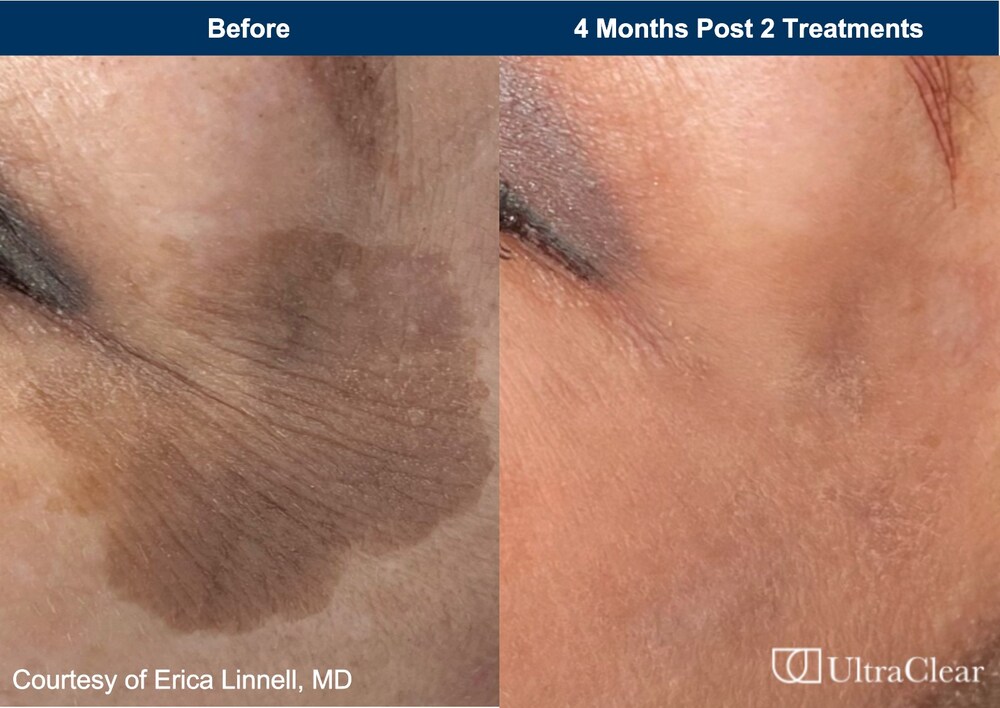
The U.S. Food and Drug Administration (FDA) has cleared Acclaro Medical’s UltraClear cold ablative fractional 2910 nm fiber laser for the treatment of benign pigmented lesions and vascular dyschromia.
The laser is already cleared for general skin resurfacing procedures and treating fine lines and wrinkles, benign age spots, scars, and skin tone.
“Any change in skin tone can be concerning or upsetting,” says Erica Linnell, MD, Founder MD of Linnell Dermatology and Aesthetics, Seattle, WA, in a news release. “With the heightened awareness of skin cancer checks and an aging population, we have seen a corresponding rise in patient visits for evaluation and treatment of their pigmented lesions. The expanded regulatory indication for the UltraClear fiber laser now paves the way for us to resurface and make a difference in improving pigmentation issues with just one to two treatments.”
Shlomo Assa, Co-Founder and President, Chief Technology Officer of Acclaro Medical, adds, “This clearance underpins our commitment to addressing the unmet needs of patients living with pigmentation disorders by improving upon the current treatment paradigm of acids, peels and passé devices. Whether troubled by benign brown lesions or red discoloration, patients can now experience UltraClear’s proven ability to rejuvenate their physical appearance with excellent results, reduced discomfort, minimal downtime and very limited risk.”