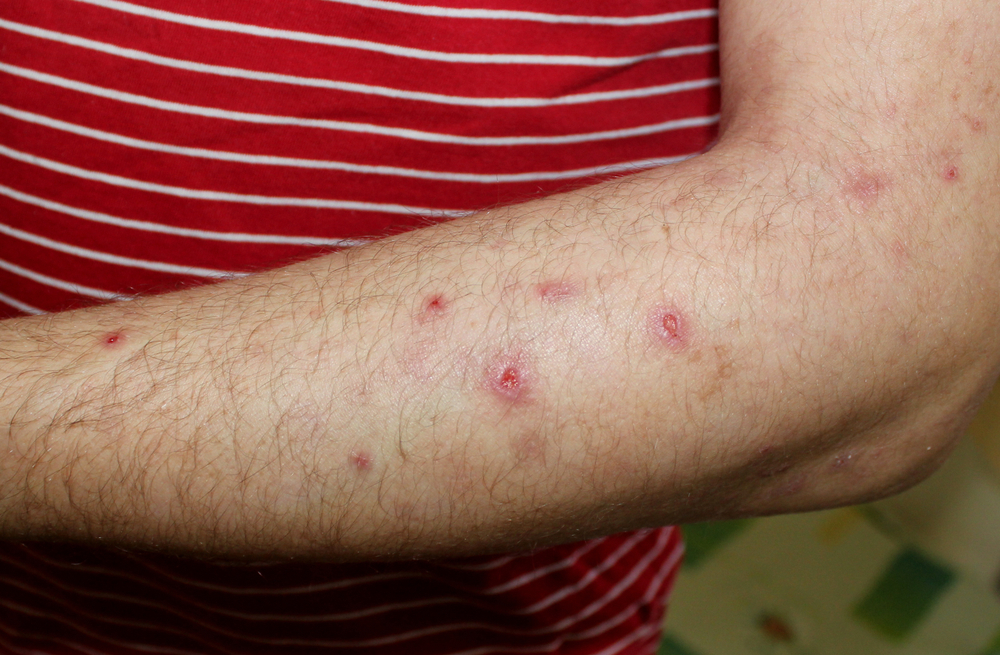
Dr. Ted Rosen discusses the FDA’s recent approval of dupilumab as the first FDA-approved treatment for adults with prurigo nodularis.
By Ted Rosen, MD, FAAD
Editor-in-Chief
Based upon the results of two phase 3 trials (PRIME, PRIME 2), the FDA has approved dupilumab (Dupixent, Sanofi Regeneron) for the treatment of prurigo nodularis in adults. Dupilumab is, of course, not a new drug per se, but this is the first drug specifically approved for this indication.
The drug was administered as an initial loading dose of 600 mg, followed by 300 mg every two weeks.
The primary endpoint in these studies was a clinically meaningful reduction in itch, defined as a 4 or greater point reduction in the Worst Itch Numeric Rating Scale (0-10 scale) at study end (24 weeks).
Additional endpoints included the proportion of patients with clear or almost-clear skin (absence or near absence of nodules), and the proportion of patients who met both the foregoing criteria.
In PRIME and PRIME 2, about 60% and 58%, respectfully, of subjects experienced a clinically meaningful reduction in itch at 24 weeks, compared to 18% and 20% for placebo. The difference in effect on itch was apparent even at 12 weeks, where 44% and 37% of dupilumab-treated patients compared to 16% and 22% of placebo-treated had a meaningful itch reduction.
A total of 48% and 45% of dupilumab-treated patients compared to 18% and 16% of placebo-treated patients achieved clear or almost-clear skin at 24 weeks.
At 24 weeks, 39% and 32% of study subjects experienced both meaningful itch reduction and clear or almost-clear skin when treated with dupilumab. This compares to 9% in both studies of those treated with placebo.
The safety profile of dupilumab when administered for prurigo nodularis was consistent with the well-known safety profile when this drug is given for atopic dermatitis. A total of 4% of dupilumab-treated subjects in these trials experienced conjunctivitis compared to 1% of placebo recipients.
Suggested Reading:
FDA. FDA approves first treatment for prurigo nodularis. FDA.gov. (current as of) September 29, 2020. Accessed January 2, 2023.