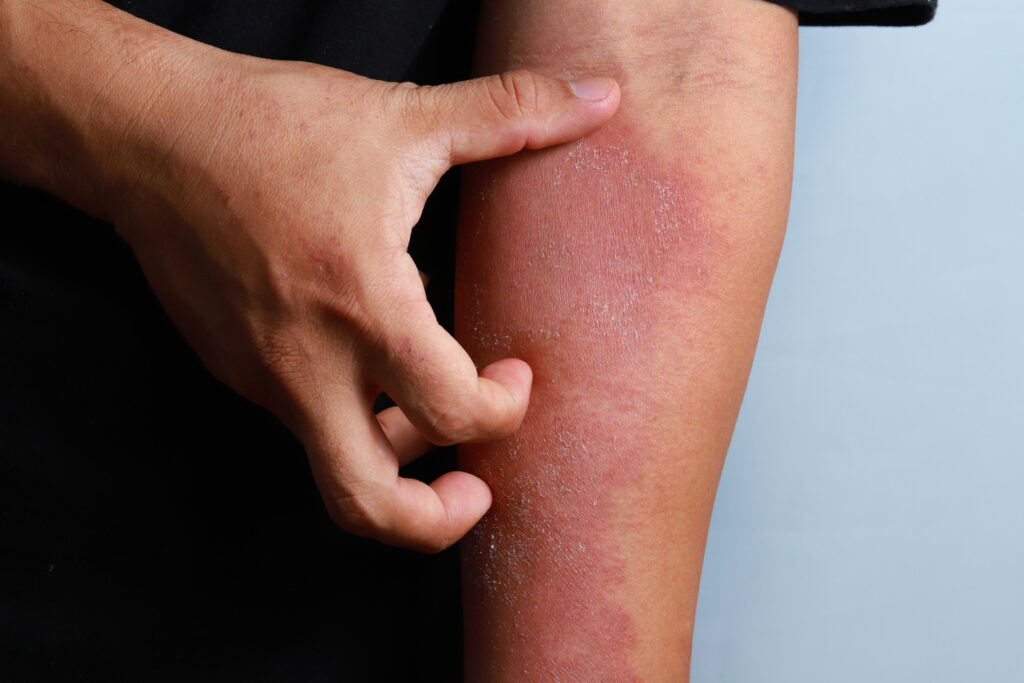
The U.S. Food and Drug Administration (FDA) has approved nemolizumab (Nemluvio, Galderma) for the treatment of patients aged 12 and older with moderate to severe atopic dermatitis, in combination with topical corticosteroids (TCS) and/or calcineurin inhibitors (TCI) when the disease is not adequately controlled with topical prescription therapies.
This action follows the recent FDA approval of nemolizumab for subcutaneous injection for the treatment of adults with prurigo nodularis in August 2024.
Nemolizumab is the first approved monoclonal antibody that specifically targets interleukin (IL)-31 receptor alpha, inhibiting the signaling of IL-31, sometimes called the itch cytokine.
This approval is based on positive results from the phase III ARCADIA clinical trial program which evaluated the efficacy and safety of nemolizumab in combination with background TCS, with or without TCI, versus placebo in combination with TCS, with or without TCI, in 1,728 patients aged 12 or older with moderate to severe atopic dermatitis.
Results demonstrated that patients treated with nemolizumab, administered subcutaneously every four weeks in combination with TCS, with or without TCI, showed statistically significant improvements on skin clearance in both co-primary endpoints. These were clearance (0) or almost-clearance (1) of skin lesions when assessed using the investigator’s global assessment (IGA) score, and achieving a 75% reduction in the Eczema Area and Severity Index (EASI) – when compared to placebo in combination with TCS, with or without TCI, after 16 weeks of treatment.
The trials also met all key secondary endpoints confirming significant responses on itch as early as Week 1, and statistically significant improvements in sleep disturbance with Nemluvio in combination with TCS, with or without TCI, when compared to placebo in combination with TCS, with or without TCI.
Overall, nemolizumab was well tolerated, and the safety profile was generally consistent between Nemluvio and placebo groups.
Experts React
“Despite currently available treatment options, atopic dermatitis continues to have a massive impact worldwide, with patients not only burdened by intense itch and recurrent skin lesions, but also potentially several associated symptoms including sleep issues, pain, anxiety, and depression,” says Jonathan Silverberg, MD, PhD, MPH, Professor of Dermatology at George Washington University School of Medicine and Health Sciences in Washington, DC, in a news release. “I look forward to being able to offer this option to atopic dermatitis patients in my practice who are seeking relief from burdensome itch and lesions.”
Peter A. Lio, MD, a Clinical Assistant Professor of Dermatology and Pediatrics at Northwestern University Feinberg School of Medicine and a partner at Medical Dermatology Associates of Chicago, IL, agrees. “Nemolizumab represents an exciting new pathway for the targeted treatment of atopic dermatitis,” he tells TDD, “Targeting IL-31 is unique to the biologics and has been shown in trials to help not only the itch, but also the cutaneous lesions of AD with a very reassuring safety profile and monthly dosing.”