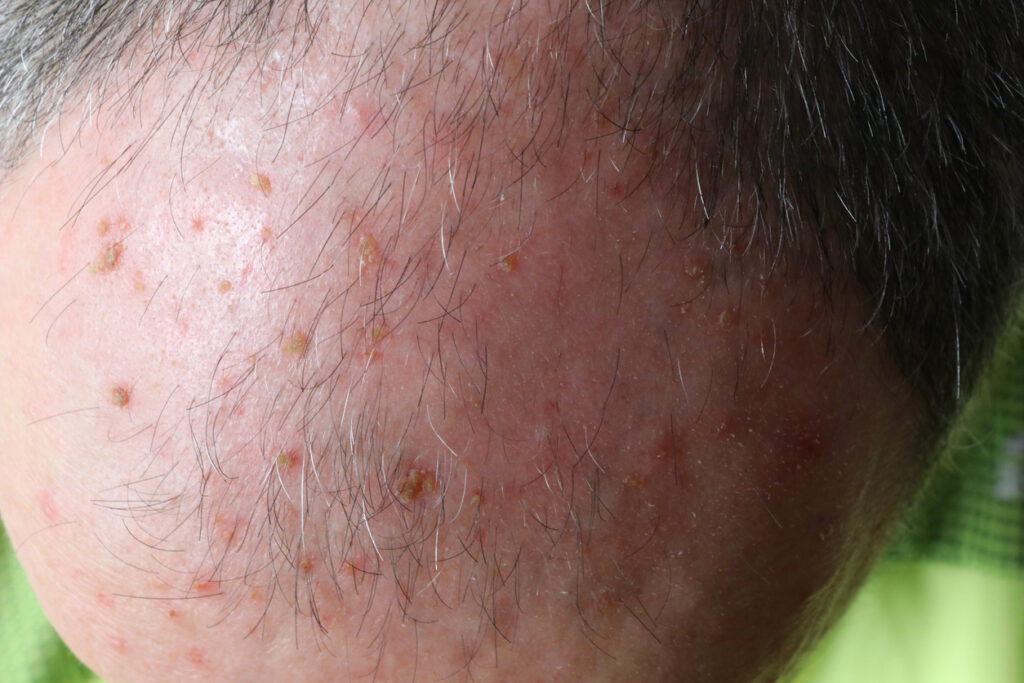
Biofrontera Inc. has installed the 100th commercial RhodoLED XL Lamp in the U.S. market.
The RhodoLED XL was approved by the U.S. Food and Drug Administration (FDA) for use in combination with aminolevulinic acid HCl (Ameluz) in 2022 and was launched in June 2024. Ameluz is used in combination with photodynamic therapy using BF-RhodoLED or RhodoLED XL lamp for the lesion-directed and field-directed treatment of actinic keratoses of mild to moderate severity on the face and scalp.
The main difference between the company’s existing BF-RhodoLED lamp and the new RhodoLED XL is the number of LED panels which allows a larger surface area to be illuminated during a single PDT treatment with Ameluz. In October 2024, the FDA approved the use of up to three tubes of Ameluz in one treatment with the BF-RhodoLED or RhodoLED XL lamps. This is up from the previous maximum of one tube.
After a three-month evaluation of the RhodoLED XL, Aaron Hoover, MD, of Front Range Dermatology in Greeley, CO, was so impressed with its performance that his practice decided to purchase not one but two units, including the 100th installed RhodoLED XL lamp. “We were thoroughly impressed by the lamp’s robust yet elegant design, as well as its exceptional maneuverability and adjustability, which made it ideal for Photodynamic Therapy (PDT) treatments in our offices,” Dr. Hoover explains in a news release. “The larger illumination area has significantly increased our patient throughput while also enhancing the overall quality of care and patient experience.” Dr. Hoover also noted the high level of satisfaction among both staff and patients with the outcomes of using Ameluz PDT in conjunction with the RhodoLED XL. “Additionally, the Biofrontera team made the purchase and transition to the XL an entirely seamless process for us,” he adds.