There’s a new over-the-counter topical eczema probiotic on the block, and it is based on a discovery by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
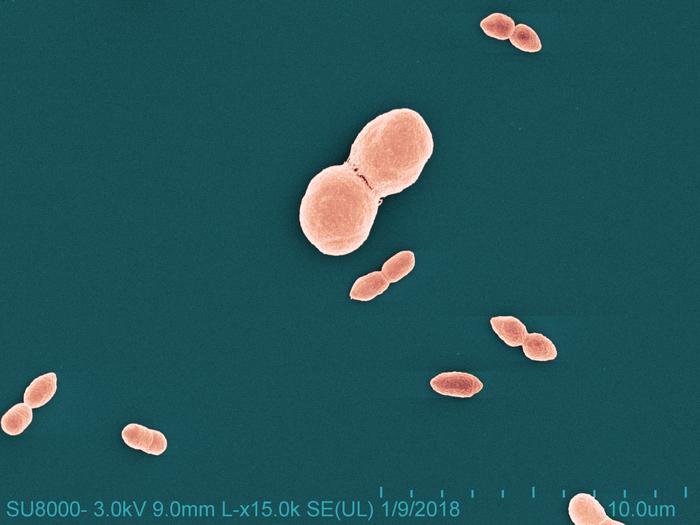
The group found that bacteria present on healthy skin called Roseomonas mucosa can safely relieve eczema symptoms in adults and children, and an R. mucosa-based probiotic formulated by Skinesa and called Defensin was just released.
Scientists led by Ian Myles, MD, MPH., chief of NIAID’s Laboratory of Clinical Immunology & Microbiology Epithelial Research Unit, found specific strains of R. mucosa reduced eczema-related skin inflammation and enhanced the skin’s natural barrier function in both adults and children.
To arrive at this finding, Dr. Myles and colleagues spearheaded a spectrum of translational research on R. mucosa. They isolated and cultured R. mucosa in the laboratory, conducted preclinical (laboratory/animal) and clinical (human) studies, and made the bacteria available for commercial, non-therapeutic development.
In Phase 1/2 open-label and Phase 2 blinded, placebo-controlled clinical studies, most people experienced greater than 75% improvement in eczema severity following application of R. mucosa. Improvement was seen on all treated skin sites, including the inner elbows, inner knees, hands, trunk, and neck. The researchers also observed improvement in skin barrier function. Additionally, most participants needed fewer corticosteroids to manage their eczema, experienced less itching, and reported a better quality of life following R. mucosa therapy. These benefits persisted after treatment ended: therapeutic R. mucosa strains remained on the skin for up to eight months in study participants who were observed for that duration.
To expand the potential use of R. mucosa, NIAID will conduct an additional clinical trial to generate further evidence on its efficacy in reducing eczema symptoms. Those data could form the basis of an application to the Food and Drug Administration (FDA)to enable the product to be regulated as a nonprescription drug and made accessible to a broader population of people with eczema.
Study results are expected in 2024. Stay tuned.