With Shawn Kwatra, MD

Dr. Shawn Kwatra discussed using medical marijuana as an adjunctive treatment for chronic itch, detailed a specific case, and called for more research.
“For many of my chronic itch patients who are miserable, their itch is managed only with off-label therapeutics with less favorable side effect profiles,” said Shawn Kwatra, MD. “We have a lot of patients that are very itchy, and there are currently no FDA-approved therapies specifically for itch as an indication.”
Because there are no approved treatments, “dermatologists and other providers are forced to use alternative and off-label agents with variable success in our clinics,” Dr. Kwatra said.
Dr. Kwatra and coauthors Natalia Fontecilla Biles, MD, Youkyung Roh, BA, and Nishadh Sutaria, BS, recently published a case of chronic itch in a 60+-year-old female patient with underlying cholestatic pruritus who was recalcitrant to standard antipruritic therapies, including topical steroids, phototherapy, capsaicin, doxepin, naltrexone, and butorphanol nasal spray.1
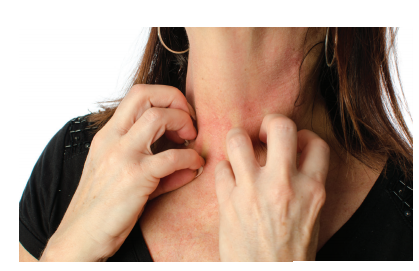
Patient-reported itch was a 10/10 on the Worst Itch Numeric Rating Scale (WI-NRS).
“The patient was really suffering, and given emerging research on the role of the endocannabinoid system in itch, we made a decision to write for medical marijuana,” said Dr. Kwatra. “In Maryland, medical marijuana is an option… so we gave a prescription and the patient went to a dispensary.”
The patient either smoked tetrahydrocannabinol (THC), 18% indica flower, or sublingually dosed a 1:1 THC-cannabinol tincture 2 nights per week.
According to Dr. Kwatra, dosing was based on medical marijuana guidelines for chronic pain, an indication that has some data behind it.
“As a provider, when you approve a patient to receive medical marijuana, [in states where it is legalized] there is already a little bit of guidance on dosing… we just followed similar guidance that’s available for [chronic] pain. Chronic pain and itch have related signaling mechanisms, so several medications indicated for pain, such as gabapentin, pregabalin, or butorphanol, can also be used off-label for chronic itch with variable success.”
WATCH — An exclusive TDD interview with Dr. Kwatra for more details on dosing, delivery, and side effects.
Over the course of treatment WI-NRS scores went from a 10/10 to a 4/10 at 5- and 12-month follow-up, and to a 0/10 at 16- and 20-month follow-up.
“The patient actually only had to use it twice weekly, and [the treatment] had a significant response in [the patient’s] itch. Immediately it fell within a few minutes of administration from… a 10/10 to a 4/10, and then over the course of therapy, [the] itch went all the way down to zero and disappeared. For us that was a really significant finding.”
There were no systemic side effects and the patient was satisfied with treatment.
“We’re really happy that the patient had such a good response because other therapies the patient had tried before had some significant side effects and, at least for this therapy, they didn’t have significant systemic side effects. [The patient was] very happy with therapy, and we were happy that we were able to fill that void for the patient.”
Dr. Kwatra indicated this case demonstrated the potential for treating itch with medical marijuana, but also acknowledged the need for clinical trial research.
“…there are many different factors to consider in terms of how to standardize the amount of medication given to patients and also the right indication,” noted Dr. Kwatra. “So at this point, more research with controlled trials are needed to determine in which situations we think it would be most effective and having that help guide therapy.”
By Eliza Cabana
Reference: 1 Roh YS, Sutaria N, Biles NF, Kwatra SG. Treatment of Chronic Pruritus With Medical Marijuana. JAMA Dermatol. Published online April 09, 2021. doi:10.1001/jamadermatol.2021.119


